Tristel ULT
Tristel ULT is a high-level disinfectant foam intended to disinfect cleaned, reusable, non-lumened ultrasound probes. Tristel ULT is intended to be used by qualified healthcare personnel. Tristel ULT does not sterilize.
Tristel ULT is a high-level disinfectant foam intended to disinfect cleaned, reusable, non-lumened ultrasound probes. Tristel ULT is intended to be used by qualified healthcare personnel. Tristel ULT does not sterilize.
Tristel ULT is a high level disinfectant foam for reprocessing ultrasound probes.
Tristel ULT can be used to high level disinfect endocavity transvaginal and transrectal probes, and skin surface transducers that may contact non-intact skin during use.
Used by professionals in 35 countries since 2008, Tristel ULT is now FDA-approved for sale in the United States.
Tristel ULT is a prescription device under 21 CFR Part 801.109, classified as a Class II device under the generic name foam or gel chemical sterilant/high-level disinfectant.

Tristel ULT is a ready to-use foam with a wipe, it does not require installation, plumbing, water or
electricity.

At 2 minutes, Tristel ULT is the fastest FDA-cleared high-level disinfectant.

Tristel ULT is compact, mobile and can be used at a site closest to the point of care.

Tristel ULT is compatible with 965 probes and transducers from leading manufacturers.

Tristel ULT is a high-level disinfectant and is effective against HPV type 16 and type 18

Tristel ULT use increases probe availability and enables healthcare staff to attend to more patients.
Tristel ULT is a device which incorporates intuitive design features, operating as ready-to-use, thus securing successful user handling and dosing of the high level disinfectant at or above the specified Minimum Recommended Concentration (MRC).
A measured dose delivery is ensured with each application.
MRC of Tristel ULT may be verified using Tristel Test Strips, semi-quantitative chemical indicators.
Tristel Test Strips detect the MRC and above giving a color indication of a “PASS” and if the concentration is below the MRC a color indication of a “FAIL”.
Minimum Recommended Concentration (MRC): ~90% v/v (~280ppm) at point of use.
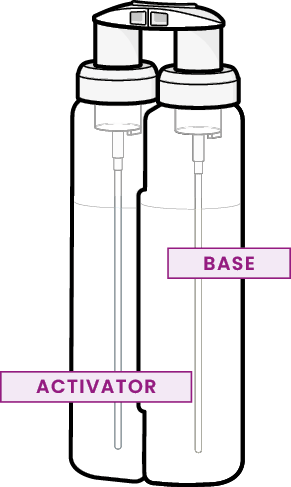
Tristel ULT is based on Tristel’s proprietary chlorine dioxide (ClO2) chemistry. Tristel ULT generates chlorine dioxide foam from two “precursor” solutions (Base Solution and Activator Solution), which are packaged separately within two individual containers and secured together within a single dispenser apparatus known as the “pump”.
Chlorine dioxide is an oxidizing biocide. It deactivates microorganisms by attacking and penetrating their cell wall, disrupting the transport of nutrients across the cell wall by inhibiting protein synthesis.
As this action occurs regardless of the metabolic state of the organism, oxidizing biocides are effective against dormant organisms and spores.
Tristel ULT is a high-level disinfectant with a contact time of 2 minutes. As a high-level disinfectant, Tristel ULT was tested for efficacy against mycobacteria, viruses, fungi and bacteria. Tristel ULT passed the AOAC Sporicidal Activity Test.
Specific testing was carried out for clinically relevant microbes.
Tristel ULT was tested for efficacy against Human Papillomavirus Type 16 (HPV16) and Type 18 (HPV18) in a study conducted by Dr. Craig Meyers at the Pennsylvania State College of Medicine, USA. The study concludes that Tristel ULT is successful at inactivating HPV16 and HPV18.1
Tristel ULT is efficacious against:
Simulated device studies with Mycobacterium terrae validated efficacy on ultrasound probes. Clinical in-use studies in US hospitals confirmed the performance of Tristel ULT in clinical settings with no organisms recovered from patient-contaminated ultrasound probes which were high-level disinfected with Tristel ULT.
1 Meyers C, Milici J, Robison R. The ability of two chlorine dioxide chemistries to inactivate human papillomavirus-contaminated endocavitary ultrasound probes and nasendoscopes. J Med Virol. 2020 Aug;92(8):1298-1302. doi: 10.1002/jmv.25666. Epub 2020 Feb 4. PMID: 31919857; PMCID: PMC7497195.
Tristel ULT has been tested and confirmed compatible with representative medical devices of leading manufacturers, including:
|
|
For a detailed model list, please refer to the Device Compatibility Summary.
Tristel ULT biocompatibility was assessed on the 100% concentrated strength and through evaluation of residues left on devices after high-level disinfection.
Tristel ULT is non-mutagenic, non-clastogenic in maturing erythrocytes, and non-oral toxic. It is classified as a slight irritant to the skin and an eye irritant in direct contact.
Any potential residues remaining have not been found irritating to dermal, vaginal and rectal tissues.
3T is a fully featured, cloud-based device reprocessing compliance platform.
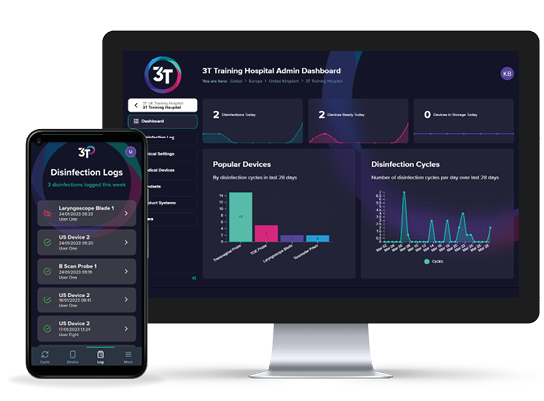
3T combines an intuitive mobile app for use at the point of care with an interactive web portal. It provides users with a valuable tool for record and traceability of device reprocessing.
3T guides users through the device reprocessing steps, tracking each event and making the record available to view in real-time.
Aggregated data from device reprocessing events are securely stored and can be found across interactive dashboards, allowing administrators to view and share customised reports.
For manual traceability, a log sheet is available to download.
Visit 3t.tristel.com to learn more about 3T.
CONTACT PARKER LABORATORIES
Email: customerservice@parkerlabs.com – Call: 973.276.9500


Tristel ULT is not dangerous for shipment and can be transported by air, road and sea without restrictions.