Game Changing High-Level Disinfection
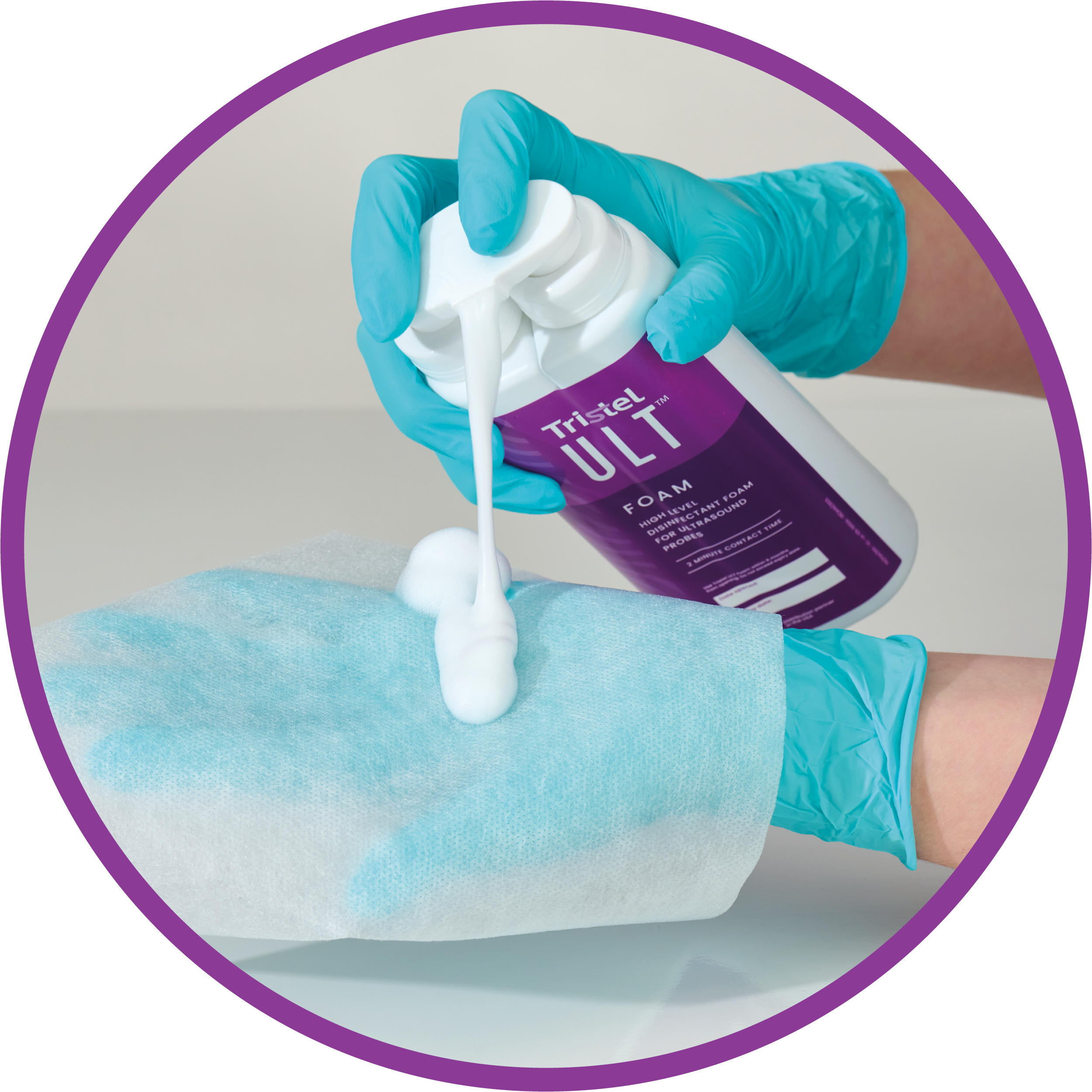
Achieve high-level disinfection at point-of care in just 2 minutes. No special equipment or facilities needed; just four pumps of Tristel’s FDA cleared CIO2 foam on a Tristel ULT dry wipe.
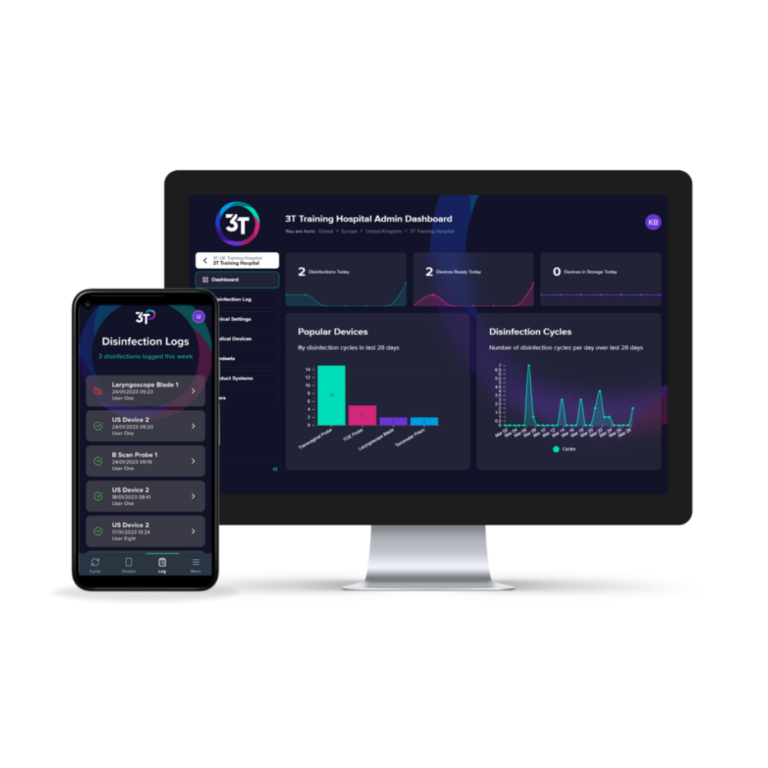
Tristel ULT can be used with Tristel 3T, a free digital traceability and training platform delivering seamless monitoring and peace of mind.
Boost efficiency and patient throughput with a cost-effective, easy-to-use solution. Compatible with 1,000+ ultrasound probes, Tristel ULT offers speed, effectiveness and versatility.
Product Code: 45-25

Applications
High-level disinfection for ultrasound probes.
Endocavity
Probes
Skin Surface Transducers
Why Choose Tristel ULT?
Tristel ULT offers an effective, fast and compliant high-level disinfection solution for ultrasound devices.
- Effective
- Compatible
- Mobile and Economical
- Compliant
-
Proven effective against a wide range of microorganisms in 2 minutes, including:
- Chlamydia trachomatis
- Neisseria gonorrhoeae
- Carbapenem resistant Klebsiella pneumoniae (CRKP)
- Extended-Spectrum beta-lactamase (ESBL) producing Escherichia coli
- Streptococcus agalactiae
- Candida albicans
- Human Immunodeficiency Virus (HIV) Type 1
- Hepatitis B Virus Surrogate Duck Hepatitis B Virus
For the full list of microorganisms Tristel ULT is effective against, please see the Microbiological Efficacy Summary.
-
Tested and proven to be compatible with the instruments of major manufacturers, including but not limited to:
-
- BD (Bard Access)
- Canon Medical Systems
- FUJIFILM Healthcare
- GE Healthcare
- Mindray
- Philips
- Samsung Healthcare
- Siemens Healthineers
- Sonoscape
- Sonosite
- Verathon
-
-
Tristel ULT can be used wherever required, without having to worry about the limitations that automated systems bring. Tristel ULT is also suitable for use in remote locations where electricity and water are not readily available.
Tristel ULT does not require investment in expensive equipment or service contracts. Four doses of foam and two Tristel ULT wipes are all it takes to achieve disinfection to the highest level.
-
Tristel ULT is a prescription device under 21 CFR Part 801.109, classified as a Class II device under the generic name foam or gel chemical sterilant/high-level disinfectant.
The ANSI/AAMI ST58 standard now recognizes chlorine dioxide foam as a high-level disinfectant—the technology behind Tristel ULT.
Product information
- Description
- Easy to Use
- Chemistry
- Microbial Efficacy
- Traceability
- Resources
- Minimum Recommended Concentration (MRC) Check
- Where to Buy
-
Description
Tristel ULT is a high level disinfectant foam for reprocessing ultrasound probes.
Tristel ULT can be used to high level disinfect endocavity transvaginal and transrectal probes, and skin surface transducers that may contact non-intact skin during use.
Tristel ULT is a prescription device under 21 CFR Part 801.109, classified as a Class II device under the generic name foam or gel chemical sterilant/high-level disinfectant.
-
Easy to Use
DispenseWipeWaitTraceRemove ResidueRefer to the User Guide for full instructions.
Chemistry
Tristel ULT utilizes our proprietary chlorine dioxide chemistry (CLO2), a well-documented and highly effective biocide that destroys microorganisms by taking electrons, ensuring they cannot develop resistance to the chemistry.
It has two separate compartments that contain 125ml of Tristel Base Solution (citric acid) and 125ml of Tristel Activator Solution (sodium chlorite). When the pump is pressed, the two solutions mix and Tristel chlorine dioxide chemistry is generated.
Our chlorine dioxide does not form hazardous by-products or halogenated compounds.
Microbial Efficacy
Tristel ULT is a high-level disinfectant with a contact time of 2 minutes. As a high-level disinfectant, Tristel ULT was tested for efficacy against mycobacteria, viruses, fungi and bacteria. Tristel ULT passed the AOAC Sporicidal Activity Test.
Specific testing was carried out for clinically relevant microbes.
Tristel ULT was tested for efficacy against Human Papillomavirus Type 16 (HPV16) and Type 18 (HPV18) in a study conducted by Dr. Craig Meyers at the Pennsylvania State College of Medicine, USA. The study concludes that Tristel ULT is successful at inactivating HPV16 and HPV181.
Simulated device studies with Mycobacterium terrae validated efficacy on ultrasound probes. Clinical in-use studies in US hospitals confirmed the performance of Tristel ULT in clinical settings with no organisms recovered from patient-contaminated ultrasound probes which were high-level disinfected with Tristel ULT.
1Meyers C, Milici J, Robison R. The ability of two chlorine dioxide chemistries to inactivate human papillomavirus-contaminated endocavitary ultrasound probes and nasendoscopes. J Med Virol. 2020 Aug;92(8):1298-1302. doi: 10.1002/jmv.25666. Epub 2020 Feb 4. PMID: 31919857; PMCID: PMC7497195.
Traceability
3T is an easy-to-use digital compliance platform that allows you to log high level disinfection cycles and track staff training all in one place.
3T combines an intuitive mobile app for use at the point of care with an interactive web portal. It provides users with a valuable tool for record and traceability of device reprocessing.3T guides users through the device record keeping steps, tracking each event and making the record available to view in real-time.
Aggregated data from device reprocessing events are securely stored and can be found across interactive dashboards.For manual traceability, a log sheet is available to download.
Resources
Minimum Recommended Concentration (MRC) Check
MRC of Tristel ULT may be verified using Tristel Test Strips, semi-quantitative chemical indicators.
Tristel Test Strips detect the MRC and above giving a color indication of a “PASS” and if the concentration is below the MRC a color indication of a “FAIL”.
Minimum Recommended Concentration (MRC): ~90% v/v (~280ppm) at point of use.
Where to Buy
CONTACT PARKER LABORATORIES
Email: customerservice@parkerlabs.com – Call: 973.276.9500TRISTEL ULT
TRISTEL TEST STRIPS
TRANSPORT
Tristel ULT is not dangerous for shipment and can be transported by air, road and sea without restrictions.