The Fight Against Antimicrobial Resistance Disinfect Responsibly
Fighting antimicrobial resistance (AMR) is critical for our global health. This article highlights the importance of implementing effective disinfection practices to prevent the spread of resistant bacteria. Discover how innovative solutions like Tristel’s chlorine dioxide high-level and sporicidal disinfectants offer a reliable defense against AMR, ensuring responsible disinfection without contributing to this growing public health crisis.
Antimicrobial resistance (AMR) is a global public health issue. In 2019, it directly caused more than 1.27 million deaths and contributed to 4.95 million others. In addition to its impact on human health, AMR also has a significant economic impact. Without changes, the costs related to healthcare and productivity losses could reach up to $1 trillion by 2050.1
Our Society Is Facing an Emergency…
Without concrete action, we risk entering a “post-antibiotic” era in which common infections will become impossible to treat.
Antibiotics Are One of the Causes of Antimicrobial Resistance
One of the main causes of AMR is the over-prescription and inappropriate use of antibiotics. The more frequently and excessively these drugs are used, the greater the risk of resistant bacteria developing. Not to mention that incorrect use of antibiotics, such as prematurely interrupting treatment, allows bacteria to survive and develop resistance.
In Addition to Antibiotics, Biocides Are Also Being Questioned
Antibiotics are not the only culprits. The use of certain biocides for medical devices and surface disinfection, in particular Quaternary Ammonium Compounds (QACs), has also been singled out.2
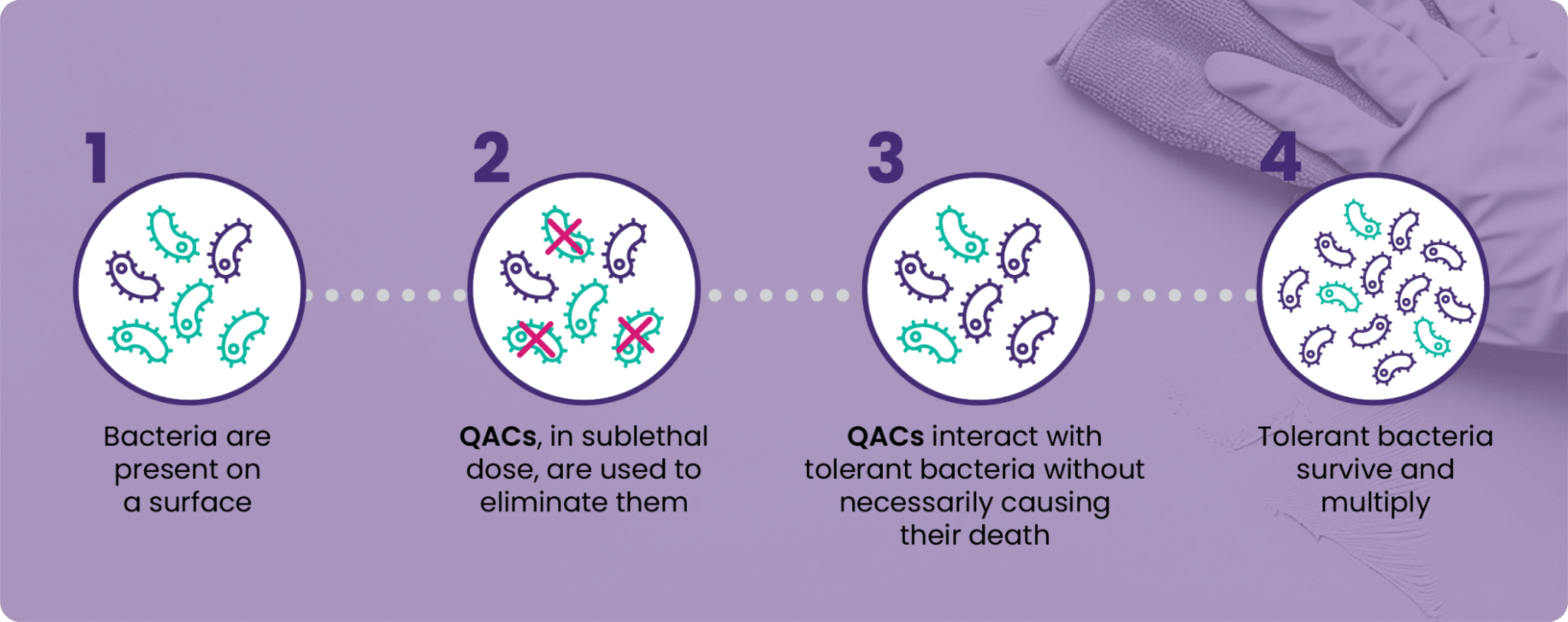
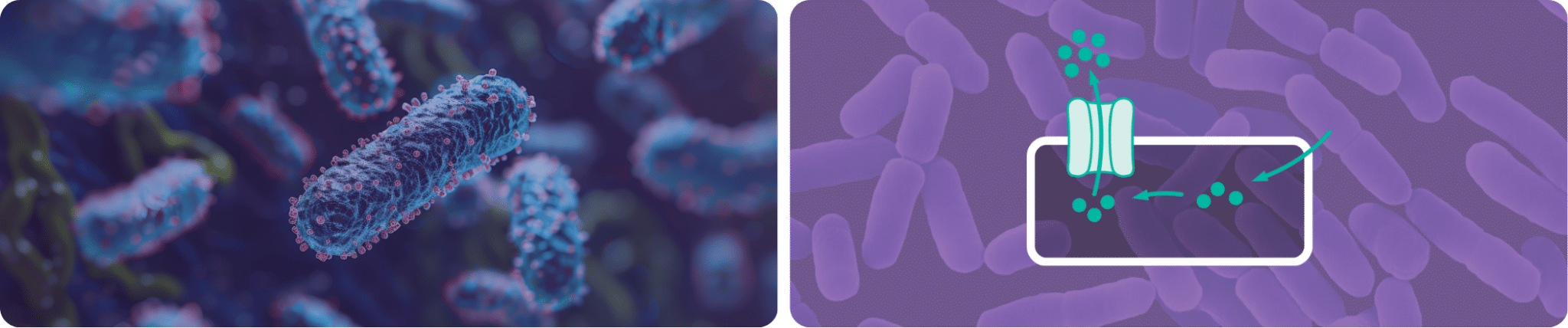
QACs are positively charged surfactants effective against a limited spectrum of micro-organisms (mainly bacteria, yeasts and enveloped viruses), which act by binding to their cell membrane. After a certain time, this wall can be penetrated, leading to a rupture of the membrane and leakage of its contents.
In simple terms, QACs work their way inside micro-organisms to cause their death. This disruption does not occur immediately or at every concentration. In many cases, it only occurs when high concentrations of QACs or combinations of QACs are used.
In all other cases, QACs can interact with tolerant micro-organisms, but not to the point of causing their death.
It is in this grey area that AMR becomes a real threat that needs to be closely monitored.
When a micro-organism is exposed to low concentrations of a biocide without being eliminated, it can develop immunity to it, a phenomenon known as acquired bacterial resistance.
Genetically, the micro-organism can manifest structures called efflux pumps, enabling the cells to regulate their internal environment by actively eliminating disinfecting agents that enter the cell. This reduces their toxic effect and helps micro-organisms to survive in an environment containing sublethal doses of disinfectants.
This acquired bacterial resistance is not limited to a single micro-organism. Bacteria that have learned to defend themselves will pass on their resistance gene to their offspring or congeners through simple contact with other micro-organisms of the same or different species.3
What Is the Link Between Tolerance to Disinfectants and Resistance to Antibiotics?
A micro-organism that has acquired resistance to a particular disinfectant agent may also use similar mechanisms to resist other antimicrobials, including antibiotics.4 This refers to co- or cross-resistance.
So if a micro-organism that used to be eliminated by a simple disinfectant spray or wipe can no longer be eliminated, the risk of it surviving and infecting a person becomes considerably higher. If this same micro-organism uses its acquired resistance mechanism to counter the effect of an antibiotic, then it risks tipping us over the edge into the era when common infections become impossible to treat.
The disinfectants that protected us to a certain extent in the past could well endanger our health system by promoting AMR.
What Can We Do to Prevent Antibiotic Resistance and Tolerance to Disinfectants?
Firstly, it is important to limit sub-inhibitory doses, i.e. doses that are not powerful enough to achieve the desired effect. This low dosage will destroy some micro-organisms, but those that survive will be less sensitive to the antimicrobials used.5,6,7 If this process were to continue, the active concentration of antimicrobials could become ineffective.
In the case of biocides for surface disinfection, solutions with variable dilutions are particularly problematic. They do not allow us to be certain that we have chosen the right concentration, i.e. one that is sufficiently powerful and effective against all the types of micro-organisms potentially present on a given surface.
Secondly, disinfectants with variable and prolonged contact times should be avoided. A long contact time with a biocide with low chemical reactivity exposes micro-organisms to sublethal concentrations. This encourages the survival of infectious micro-organisms whose susceptibility to antimicrobials is reduced.
To avoid the risk of resistance, it is, therefore, preferable to turn to solutions with a single dilution rate and proven efficacy against the full spectrum of micro-organisms in a short, uniform contact time.
Tristel’s high-level and sporicidal disinfectants and their chlorine dioxide (ClO₂) chemistry stand out in the chemical world for their effectiveness and mode of action. Tristel ClO₂ is in fact an effective biocide against bacteria, viruses, protozoa, yeasts, fungi, mycobacteria and bacterial spores.
Unlike QACs, the ability of Tristel ClO₂ to eliminate a full spectrum of micro-organisms is well established, without the need for concentration adjustment by users and without variable or prolonged contact times. Elimination is almost immediate (between 30 seconds and 5 minutes depending on the product) and is achieved at a single concentration rate (between 100ppm and 200ppm depending on the product).
Is Using a Tristel High-Level and Sporicidal Disinfectant Excessive to Treat All Infectious Risks, Even Low-Level Ones?
Daily use of Tristel ClO₂ does not contribute to AMR. In addition to its full spectrum of efficacy in a short, uniform contact time and at a single concentration rate, the biocidal activity of Tristel ClO₂ is distinguished by its mode of action, oxidation.
Tristel ClO₂ is an oxidant that kills pathogens by exchanging electrons, sequestering them from the micro-organism’s vital structures such as cell walls, membranes, organelles and genetic material. It is this exchange that causes a molecular imbalance, leading to the certain death of the micro-organisms.
Unlike QACs, which only manage to eliminate them under certain conditions, Tristel ClO₂ always destroys them, even with repeated use.8,9
The daily use of Tristel ClO₂ as a high-level and sporicidal disinfectant does not contribute to the development of AMR. On the contrary, it could well be a major control solution to combat this public health concern.
2 Boyce, J. M. (2023). Quaternary ammonium disinfectants and antiseptics: Tolerance, resistance, and potential impact on antibiotic resistance. Antimicrobial Resistance & Infection Control, 12(32). https://doi.org/10.1186/s13756-023-01241-z
3 Vega, N. M., & Gore, J. (2014). Collective antibiotic resistance: Mechanisms and implications. Current Opinion in Microbiology, 21, 28-34. https://doi.org/10.1016/j.mib.2014.09.003
4 Ortega Morente, E., Fernández-Fuentes, M. A., Grande Burgos, M. J., Abriouel, H., Pérez Pulido, R., & Gálvez, A. (2013). Biocide tolerance in bacteria. International Journal of Food Microbiology, 162(1), 13-25. https://doi.org/10.1016/j.ijfoodmicro.2012.12.028
5 Walsh, S. E., Maillard, J. Y., Russell, A. D., Catrenich, C. E., Charbonneau, D. L., & Bartolo, R. G. (2003). Development of bacterial resistance to several biocides and effects on antibiotic susceptibility. Journal of Hospital Infection, 55(2), 98-107.
6 Voumard, M., Venturelli, L., Borgatta, M., et al. (2020). Adaptation of Pseudomonas aeruginosa to constant sub-inhibitory concentrations of quaternary ammonium compounds. Environmental Science: Water Research & Technology, 6(4), 1139-1152.
7 Soumet, C., Meheust, D., Pissavin, C., et al. (2016). Reduced susceptibilities to biocides and resistance to antibiotics in food-associated bacteria following exposure to quaternary ammonium compounds. Journal of Applied Microbiology, 121(5), 1275-1281.
8 Noszticzius, Z., Wittmann, M., Kály-Kullai, K., Beregvári, Z., Kiss, I., Rosivall, L. and Szegedi, J. (2013). Chlorine Dioxide Is a Size-Selective Antimicrobial Agent. PLoS ONE, 8(11), p.e79157. https://doi.org/10.1371/journal.pone.0079157
9 Block, S., Knapp, J., & Battisti, D. (2001). Disinfection, sterilization, and preservation. In S. Block (Ed.), Disinfection, sterilization, and preservation (5th ed., pp. 215-227). Philadelphia, PA: Lippincott Williams & Wilkins.