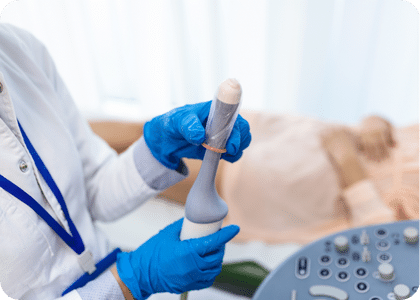
Is your decontamination process complete?
The emergence of antibiotic-resistant organisms like Methicillin-resistant Staphylococcus aureus (MRSA), and the resilience of viruses such as Human papillomavirus (HPV), which have been linked to a significant percentage of cancers1, highlight the critical need for appropriate disinfectant products. |
 |
What do I need to know?
The key question to consider is whether your decontamination procedures align with the official guidelines for ultrasound. As product options and methods increase on the market, different concepts of cleaning, disinfection, and decontamination have emerged, making it hard to determine the answer. It’s important to understand the crucial differences between available products and methods when evaluating a decontamination process.
To help you better understand, we’ve put together five simple comparisons so you can confidently choose the most effective and appropriate solutions for safely decontaminating your ultrasound devices.
References
| Ultrasound is changing. More ultrasound probes are in use today than ever before, supporting quick and effective patient diagnosis and imaging. For point-of-care ultrasound (POCUS) to work, the ultrasound devices need to be in the same location as the patient, and the decontamination products need to keep up.
POCUS presents a challenge to high-level disinfection machines. Machines are tied to a specific location as they might need a power supply, or a water source, and often they are only certified for use in that location due to staff and patient safety, they take up considerable space, and have detailed setup requirements. For your ultrasound probes, choose a method that is:
|
 |
| We believe in transparency. Every claim we make can be substantiated.
When reading studies that compare ultrasound decontamination processes, ask yourself the following:
Scientific data is the foundation of Tristel decontamination products. We combine powerful chemistry with proven test methods.
|
 |
| Decontamination is the procedure that combines both cleaning and disinfection, with mandatory thorough cleaning to be performed prior to any disinfection process. As high-level disinfection machines only address part of the decontamination process, the only possible option for non-immersible parts, such as the probe cable and plug, is manual wiping.2
Cleaning is achieved by combining a chemical with detergent quality with manual action. Manufacturers of high-level disinfectants should instruct the user to clean prior to disinfection. How can the user determine an appropriate cleaning agent to use? The interaction between the cleaning agent and the disinfection process must be considered. Rinsing between these steps will be mandatory, unless both the cleaning agent and the disinfectant are confirmed compatible by the manufacturer. Why not choose a decontamination product that accounts for the cleaning step? |
 |
References
| Cleaning is mandatory prior to disinfection, to ensure organic or inorganic soiling such as blood, mucous, tissue and ultrasound gel are removed. Otherwise, they may impede the microbicidal activity of a disinfectant chosen.3
Using manual methods such as wiping to clean medical devices is widely accepted. Many automated methods available are solely meant to high-level disinfect, and naturally lack the capability of cleaning which is necessary for effective decontamination.4 Guidelines do not recommend machine processes unless an appropriate cleaning step using a manual wipe is implemented beforehand.5 Manufacturers of high-level disinfectants should instruct the user to clean prior to disinfection. How can the user determine an appropriate cleaning agent to use? The interaction between the cleaning agent and the disinfection process must be considered. Rinsing between these steps will be mandatory, unless both the cleaning agent and the disinfectant are confirmed compatible by the manufacturer. Why not choose a decontamination product that accounts for the cleaning step? |
 |
References
| All decontamination products require multiple factors to be accounted for during the development process. Often high-level disinfection machines prioritise convenience above all else. But is this in the patients’ best interest?
Every decontamination process should be evaluated against its intended use together with ease of use, efficacy data, safety profile, device compatibility and more. Studies have shown that UV-C light employed by machines lacks the ability to reach areas of a device that are shadowed by indentations.6 Any areas the light cannot reach will not be adequately disinfected.7 As an infection prevention company with over 30 years of experience, Tristel provide extensively evaluated decontamination systems which address all the important performance criteria equally. |
 |
References
Download Resource Pack

Be Part of Our Community!
