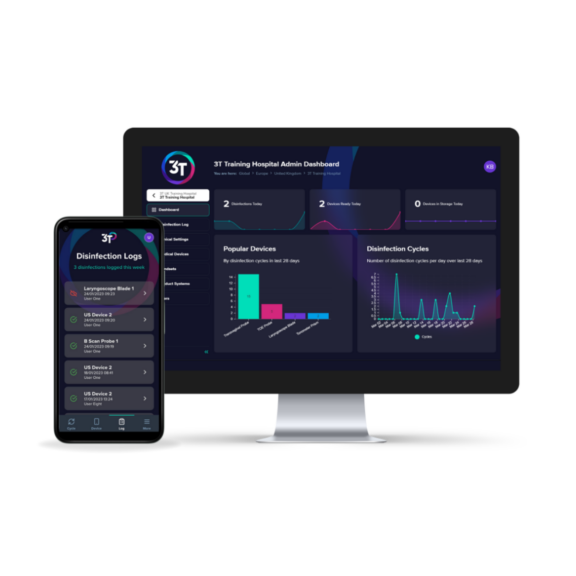
Transvaginal Ultrasound (TVUS) probes are pelvic ultrasound devices that use high-frequency sound waves for the creation of images1. They enable examinations of female reproductive organs such as the vagina, cervix, uterus, fallopian tubes, and ovaries, helping to identify and diagnose conditions2. These examinations are internal and involve inserting the probe into the vaginal canal3.
Why do TVUS probes require high-level disinfection?
1. Infection Control Guideline Endorsement
When recommending methods of disinfection or sterilisation, infection control guidelines follow the Spaulding classification. The classification is based on the infection risk to patients taking into account the intended use of the device4. When entering the patient, TVUS probes contact the intact mucous membrane of the cervix and vaginal wall. Therefore, regulatory bodies recommend high-level disinfection of TVUS probes after use based on the Spaulding Classification5.
| PATIENT CONTACT | DEVICE CLASSIFICATION | DECONTAMINATION METHOD |
| Intact skin | Non-critical | Low or intermediate-level disinfection |
| Mucous membranes or non-intact skin | Semi-critical | High-level disinfection |
| Sterile areas of the body including blood contact | Critical | Sterilisation |
The Spaulding Classication
2. Micro Perforation & Probe Cover Leakage Risks
The use of probe covers is endorsed by global guidelines to minimise contamination when performing TVUS procedures6-15. Contamination can still occur from micro perforations, partial and complete breaks of the cover during use, or incorrect placement of the cover on the probe (see Figure 1.). Some commercially produced ultrasound probe covers have unacceptably high leakage rates of up to 81%, and are not a reliable barrier against infectious agents, particularly viruses16.
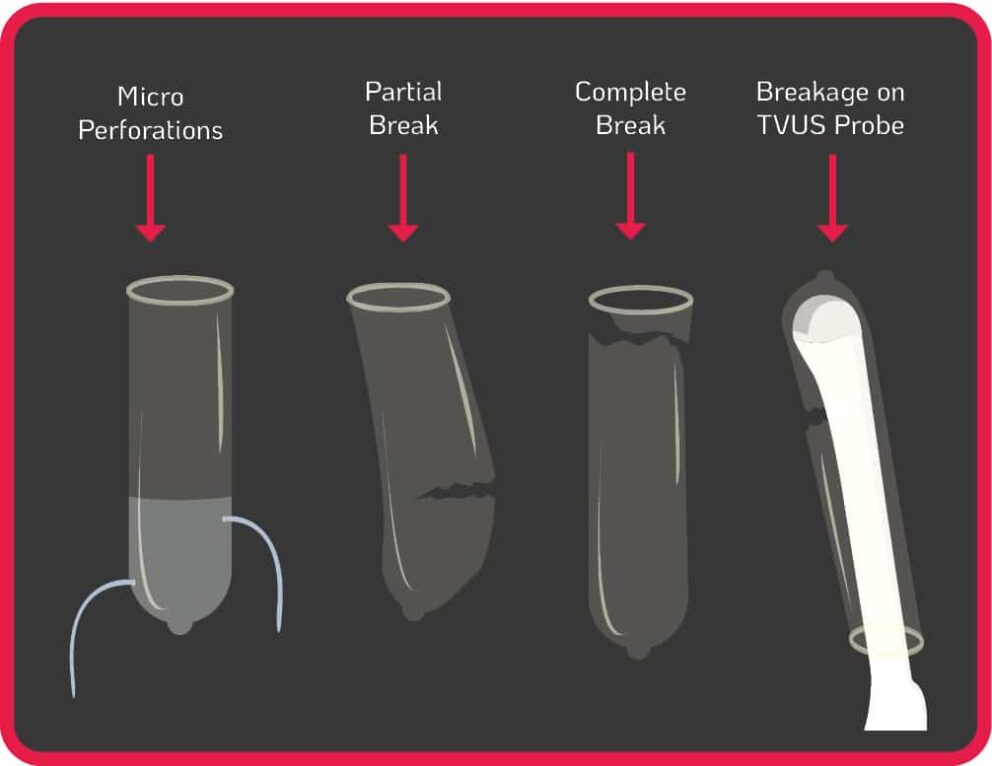
Contamination can remain even when a TVUS probe is covered and disinfected with an intermediate or low-level disinfectant17-20. If a TVUS probe is insufficiently disinfected in between patient use, or the cover was damaged/incorrectly placed (see Figure1.), nosocomial transmission can occur from patient to patient, or from patient to healthcare worker. The use of disinfectants with insufficient efficacy on medical devices will not reduce the contamination to a safe level. Pathogens can also persist for prolonged periods if a surface or device is not sufficiently disinfected. For example, Human Papillomavirus (HPV) can survive on surfaces for up to seven days21.
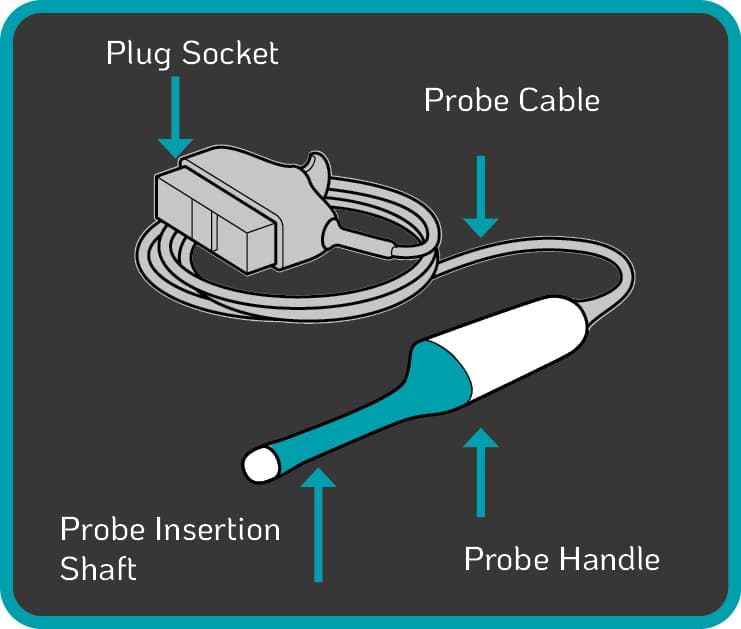
3. TVUS Probe Cable and Plug Contamination
TVUS probe components at risk of blood and microbial contamination include:
- the insertion shaft, which enters the patient22, 23
- the handle of the device24, 25
- the cable and plug26, 27
- the probe holder
High-level disinfection will prevent the spread of harmful pathogens from one patient to another while also safeguarding healthcare professionals during procedures.
Some automated disinfection units are only capable of disinfecting the insertion shaft and handle of the TVUS probe. The Tristel Trio Wipes System offers high-level disinfection for all parts of the TVUS probe including the probe, holder, plug and cable.
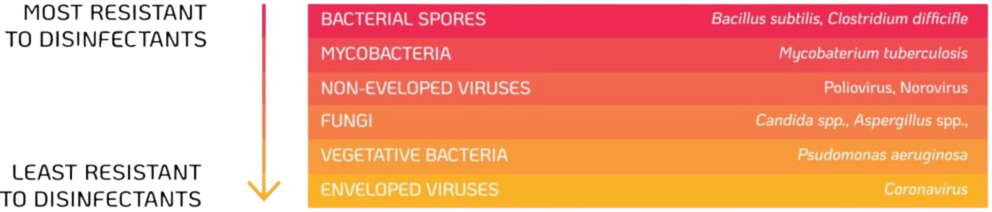
4. Risk of Bacterial Spores from the Environment
Bacterial spores such as Bacillus subtilis are common environmental contaminants28. TVUS probes are not required to be stored or decontaminated in a sterile area, which could cause the devices to become recontaminated. Bacterial spores are considered the most resistant microorganisms to disinfectants and sterilisation is needed to destroy high levels of spores. The high temperatures and harsh chemicals used during sterilisation can cause damage to the probe, therefore an alternative method of high-level disinfection should be used.
Reduces the Risk of Transmission of Infection
Scientific research by Meyers et al., (2014) demonstrated that high-level disinfectants included in worldwide decontamination guidelines are not effective at destroying HPV29. Until recently it has not been possible to test the efficacy of disinfectants against native HPV. In the absence of available methods, regulatory authorities recommend testing against the surrogate polyomavirus SV40, which is used as an indicator of efficacy against HPV. However, the resistance profiles of the two viruses in comparison against disinfectants have not been studied. This means that efficacy against the surrogate polyomavirus SV40 does not necessarily imply efficacy against HPV30. The Society for Maternal-Fetal Medicine (2020) has recently published a patient safety guideline on reducing the risk of transmitting infection by TVUS examinations, recommending the following:
- The application of single-use sterile probe covers for every TVUS examination31.
- The use of sterile single-use ultrasound gel packets32.
- Cleaning, i.e. the removal of gross contamination like gel and debris which can reduce disinfectant efficacy, on TVUS probes after each examination33.
- High-level disinfection using an agent with proven efficacy against HPV34.
TRISTEL TRIO & DUO ARE THE ONLY DISINFECTANTS PROVEN EFFECTIVE AGAINST HPV TYPES 16 & 18 IN 30 SECONDS
A new scientific study by Meyers, et al. (2020) demonstrates that the Tristel Trio Wipes System possess efficacy against infectious HPV types 16 and 18 on an endocavity ultrasound probe in 30 seconds35.

Our Products
References:
1, 2, 3 NHS. 2018. Ultrasound Scan. [online] Available at: <https://www.nhs.uk/conditions/ultrasound-scan/> [Accessed 3 October 2020].
4, 5 Centers for Disease Control and Prevention (2008) Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008 Available at: https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines-H.pdf [Accessed 3 October 2020]
6 Abramowicz JS, Evans DH, Fowlkes JB, Marsal K, TerHaar G, on behalf of the WFUMB Safety Committee. Guidelines for cleaning transvaginal ultrasound transducers between patients. Ultrasound in Med & Biol, 2017; 43(5):1076-1079.
7 EFSUMB (European Federation of Societies for Ultrasound in Medicine and Biology) -2017; http://www.efsumb.org/safety/resources/2017-probe_cleaning.pdf.
8 Prevention Du Risque Infectieux Associe Aux Actes D’echographie Endocavitaire https://solidarites-sante.gouv.fr/IMG/pdf/fiches_sondes.pdf.
9 Nyhsen, C., Humphreys, H., Koerner, R., Grenier, N., Brady, A., Sidhu, P., Nicolau, C., Mostbeck, G., D’Onofrio, M., Gangi, A. and Claudon, M., 2017. Infection prevention and control in ultrasound – best practice recommendations from the European Society of Radiology Ultrasound Working Group. Insights into Imaging, 8(6), pp.523-535.
10 Guidelines for reprocessing ultrasound transducers AJUM, 2017, 20;1:30-40.
11 BMUS / SOR (British Medical Ultrasound Society / Society and College of Radiology) -2017; https://www.bmus.org/policies-statements-guidelines/professional-guidance/guidelines-for-professional-ultrasound-practice/
12 Irish Health Service Executive (HSE) Quality Improvement Division – Decontamination Safety Programme (2017) HSE guidance for decontamination of semi‐critical ultrasound probes; Semi‐invasive and Non‐invasive Ultrasound Probes QPSD-GL-028-1 http://wwwhseie/eng/about/Who/QID/nationalsafetyprogrammes/decontamination/Ultrasound-Probe-Decontamination-Guidance-Feb-17pdf
13 Health Facilities Scotland Decontamination Services (2016) NHS Scotland guidance for decontamination of semi-critical ultrasound probes; semi-invasive and non-invasive ultrasound probes http://wwwhpsscotnhsuk/documents/hai/infectioncontrol/guidelines/NHSScotland-Guidance-for-Decontaminationof-Semi-Critical-Ultrasound-Probespdf
14 Guidelines for Reprocessing Ultrasound Transducers by the Australasian Society for Ultrasound in Medicine and the Australasian College for Infection Prevention and Control (2017) AJUM 20 (1) http://onlinelibrary.wiley.com/doi/10.1002/ajum.12042/epdf
15 Rutala WA, Weber D. Reprocessing semicritical items. Am J Infect Control. 2016;44:e53–e62. doi: 10.1016/j.ajic.2015.12.029.
16 Hamm, R., Combs, C. and Davidson, C., 2020. Society for Maternal-Fetal Medicine Special Statement: Reducing the risk of transmitting infection by transvaginal ultrasound examination. American Journal of Obstetrics and Gynecology, 223(3), pp.B2-B6.
17 M’Zali, F., Bounizra, C., Leroy, S., Mekki, Y., Quentin-Noury, C., Kann, M. (2014) ‘Persistence of MicrobialContamination on Transvaginal Ultrasound Probes despite Low-Level Disinfection Procedure’, PLoS ONE, vol. 9, no. 4 [Online]. DOI: 10.1371/journal.pone.0093368 (Accessed 17 June 2018).
18 Ma, S., Yeung, A., Chan, P., Graham, C. (2014) High level disinfection reduces HPV contamination of transvaginal sonography probes in the emergency department [Online]. Available at: https://emj.bmj.com/content/30/6/472.responses#high-level-disinfection-reduces-hpv-contamination-of-transvaginal-sonography-probes-in-the-emergency-department (Accessed 26 July 2018).
19 Casalegno, JS., Carval, K., Eibach, D., Valdeyron, ML., Lamblin, G., Jacquemoud, H., Mellier, G., Lina, B., Gaucherand, P., Mathevet, P., Mekki1, Y. (2012) ‘High Risk HPV Contamination of Endocavity Vaginal Ultrasound Probes: An Underestimated Route of Nosocomial Infection?’, PLoS ONE, vol. 7, no. 10 [Online]. DOI: doi:10.1371/journal.pone.0048137 (Accessed 23 June 2018).
20 Strauss, S., Sastry, P., Sonnex, C., Edwards, S, Gray, J. (2002) ‘Contamination of environmental surfaces by genital human papillomaviruses’,
21 Roden, R., Lowy, D., Schiller, J. (1997) ‘Papillomavirus Is Resistant to Desiccation’, The Journal of Infectious Diseases, vol. 176, no. 5., pp. 1076-1079 [Online] DOI: https://doi.org/10.1086/516515
22, 24, 26 Westerway, S. C., Basseal, J. M., Brockway, A., Hyett, J. A., Carter, D. A. (2016) ‘Potential Infection Control Risks Associated with Ultrasound Equipment – A Bacterial Perspective’, Ultrasound in Medicine & Biology [Online] DOI: 10.1016/j.ultrasmedbio.2016.09.004 (Accessed 25 October 2018).
23, 25, 27 Keys, M., Sim, B., Thom, O., Tunbridge, M., Barnett, A., Fraser, J. (2015) ‘Efforts to Attenuate the Spread of Infection (EASI): a prospective, observational multicentre survey of ultrasound equipment in Australian emergency departments and intensive care units’, Critical care and resuscitation, vol. 17, no. 1, pp. 43-46 [Online]. Available at: https://www.ncbi.nlm.nih.gov/pubmed/25702761 (Accessed 04 April 2019).
28 Allos, B.M., Blaser, M.J., Platts-Mills, J. and Kosek, M., 2014. Campylobacter Species – Infectious Disease and Antimicrobial Agents. [online] Antimicrobe.org. Available at: http://www.antimicrobe.org/new/b91.asp [Accessed 5 August 2020].
29, 30 Meyers, J., Ryndock, E., Conway, M., Meyers, C. and Robison, R., 2014. Susceptibility of high-risk human papillomavirus type 16 to clinical disinfectants. Journal of Antimicrobial Chemotherapy, 69(6), pp.1546-1550.
31, 32, 33, 34 Health Facilities Scotland Decontamination Services (2016) NHS Scotland guidance for decontamination of semi-critical ultrasound probes; semi-invasive and non-invasive ultrasound probes http://wwwhpsscotnhsuk/documents/hai/infectioncontrol/guidelines/NHSScotland-Guidance-for-Decontaminationof-Semi-Critical-Ultrasound-Probespdf
35 Meyers, C., Milici, J., Robison, R. (2020) ‘The Ability of Two Chlorine Dioxide Chemistries to Inactivate Human Papillomavirus-contaminated Endocavitary Ultrasound Probes and Nasendoscopes’. Published IN THE JOURNAL OF MEDICAL VIROLOGY [Online] Available at: bit.ly/HPVARTICLE