In healthcare settings, high-level disinfection (HLD) is essential for patient safety, but its success depends on a critical first step: cleaning. This manual process removes visible soil and organic material, which, if left behind, can compromise disinfection and allow harmful microorganisms to persist.
Importantly, all major manufacturers, both manual and automatic, agree: cleaning is the most vital step in the HLD process. It’s universally recognised as the foundation for consistent, effective decontamination.
What does the guidance say?
Thorough cleaning is essential before high-level disinfection, as any remaining organic or inorganic material can shield microorganisms and reduce disinfectant efficacy. Most commonly, cleaning involves applying a detergent-based chemical via a wipe, either pre-impregnated or combined with a dry substrate. The CDC defines this as using “fluid and friction” 1.
These practices are backed by industry guidance. In Europe, ISO 15883 outlines the standards for washer-disinfectors, detailing requirements for cleaning, efficacy, traceability, and compatibility across various medical device types.
How does disinfection differ across devices?
Medical devices in healthcare vary in their tolerance to heat, which determines the appropriate cleaning and disinfection approaches based on their design and clinical risk. Broadly, they fall into three categories:

Heat-tolerant-instruments, such as surgical instruments and some rigid endoscopes, are manually cleaned and then sterilised using steam or hydrogen peroxide plasma.
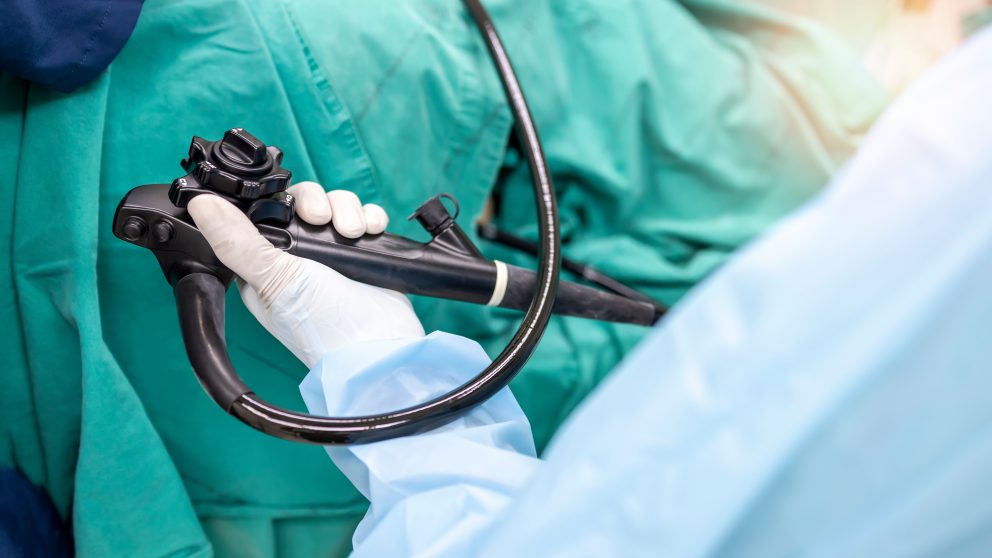
Heat-sensitive, multi-channelled devices, like flexible endoscopes need manual cleaning and lumen irrigation, followed by automated high-level disinfection in an Automated Endoscope Reprocessor (AER).
Heat-sensitive, non-lumened devices, including ultrasound probes and endoscopes, are manually cleaned and then disinfected using chemical agents or UV-C light.
Despite these differences, thorough cleaning is a universal requirement, it’s the foundation for safe and effective device reprocessing.
Are non-lumened devices easier to clean?
Compared to complex, multi-channelled endoscopes, ultrasound probes are simpler to clean and disinfect, thanks to their non-lumened design and smaller surface area.
However, endocavity probes still feature indentations, such as those for needle guides and around the tip and base that can trap contamination and pose a risk for biofilm formation if not properly cleaned.
That said, the surface area of the insertable portion of an endocavity probe is no larger than an A5 sheet of paper. With proper training and attention to detail, cleaning can be both thorough and efficient, ensuring the device is safe for high-level disinfection.
Can automation replace manual cleaning?
Technologies like UV-C light and sonicated hydrogen peroxide systems offer high-level disinfection for ultrasound probes, but they don’t perform cleaning. Instead, they rely on the cleaning step being done manually, outside their process control, a critical dependency that’s often overlooked.
Despite this, many manufacturers leave the choice of cleaning method to the end user, which introduces risk. That’s why every HLD manufacturer should specify a validated cleaning procedure for two key reasons:
- To avoid incompatible products: Without guidance, users may choose unsuitable cleaners, like quat-based wipes, which can damage probes when followed by hydrogen peroxide vapour.
- To ensure cleaning isn’t skipped: Especially with UV-C, shadowing from residual soil, gel, or even water can block the UV light and compromise disinfection.
Clear, validated cleaning protocols are essential to ensure safety and device integrity.
What is shadowing and why does it matter?
Shadowing occurs when anything blocks UV-C light from reaching the surface of a medical device, compromising disinfection. It can be caused by leftover gel, mucosal matter, cleaning product residue, or even water droplets after rinsing.
Indentations, like those on Endocavity probes, are especially vulnerable if not carefully cleaned. If a probe is still dirty, UV light won’t clean it. That’s why cleaning isn’t optional, it’s a non-negotiable prerequisite for effective disinfection.

UV-C Disinfection: What You Need to Know
Learn about UV‑C disinfection uncertainties, from lack of standards and material damage to cleaning and shadowing issues – and why careful evaluation is essential.
Choosing a validated, end-to-end reprocessing solution
When reprocessing ultrasound probes, the safest approach is one that includes validated cleaning, disinfection, and traceability. If the cleaning step is prescribed or integrated into a complete system, users can trust that the complete process has been tested for effectiveness.
Best practice is to use a cleaning agent that:
- Is CE or UKCA marked for medical devices
- Supports traceability
- Has device manufacturer approval
- Has compatibility with your chosen high-level disinfect
These markers confirm product quality, regulatory compliance, and compatibility. Standards like ISO 15883 also emphasise process monitoring, adding control and confidence to the decontamination workflow.
All of this leads to a clear and consistent message: cleaning is the most important step in any decontamination process, and it’s a manual one. It’s a responsibility we’ve trusted people with since the earliest days of infection prevention and control, and it remains central to patient safety today.
Manual wiping isn’t just a routine task. It’s a critical action that determines the success of every disinfection process. Without it, no disinfectant can perform effectively. And while automation has brought efficiency and consistency, the human hand remains unmatched in its precision, adaptability, and reliability.
With the right training, validated products, and clear protocols, manual cleaning continues to deliver effective outcomes, proving that our hands are still the most advanced tools in infection control.
References:
1 Centers for Disease Control and Prevention. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Updated June 2024. Available from: https://www.cdc.gov/infection-control/media/pdfs/Guideline-Disinfection-H.pdf.