Biofilms represent a formidable challenge in the ongoing battle against healthcare-associated infections (HAIs), as they render pathogens more tolerant to conventional cleaning and disinfection methods. Tristel chlorine dioxide products have been specifically tested for their effectiveness against both wet and dry biofilms.
The Challenge of Biofilms in Healthcare
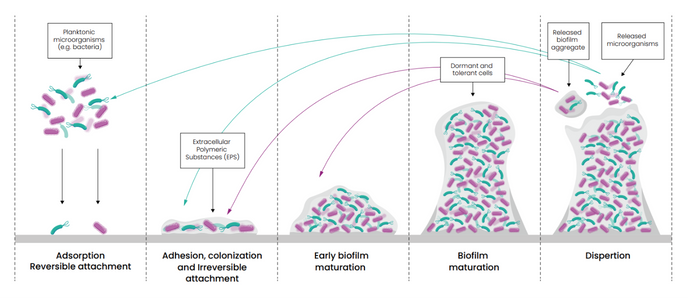
Biofilms are structured microbial communities encased in a self-produced matrix, often found clinging to surfaces in healthcare environments. Once formed, they become difficult to eradicate. This is not just a cleaning issue, it’s a patient safety issue. Biofilms act as a reservoir for multi-drug resistant organisms (MDROs), shielding them from antimicrobials whilst also increasing the potential for horizontal gene transfer, accelerating the spread of resistance.
Bacteria living in a biofilm exhibit a 10 to 1,000-fold increase in resistance to antibiotics compared to their planktonic counterparts.
Studies suggest that up to 65% of all microbial infections, and 80% of chronic infections involve biofilms. Biofilms can lead to persistent infections, increased resistance to treatments, and a heightened risk of cross-contamination. Their presence on medical equipment, environmental surfaces, and within environments such as water systems can also contribute to hospital-acquired infections (HAIs), posing a serious risk to patient safety.1,2
Are All Biofilms the Same?
While all biofilms represent a community of microorganisms encased in a protective matrix, their composition and characteristics can vary. They can consist of bacteria, yeasts and fungi, and even viruses. They typically consist of one or more different species of organisms and can persist on a surface for many hours.
Wet biofilm is a type of biofilm that forms in moist environments, where microorganisms thrive due to the presence of water and available nutrients. These microorganisms secrete a slimy layer of extracellular polymeric substance (EPS) containing polysaccharides, proteins, and lipids, embedding themselves in a protective matrix. Typically, they will consist of gram-negative bacteria such as Pseudomonas aeruginosa. In healthcare, wet biofilms can develop on and within the channels of reusable medical devices, in water lines, and on sinks, showers, and toilets and their surrounding surfaces.
Dry biofilm comprises microorganisms that form in dry or low-moisture and nutrient-deficient environments. Due to these harsh conditions, microorganisms within a developed dry biofilm tend to be more resilient. Unlike wet biofilms, dry biofilms are found on surfaces with minimal moisture, such as on medical equipment or dry environmental surfaces. These biofilms can be challenging to detect and remove, as they are often more resistant to cleaning and disinfection efforts due to their dry state.
Chlorine dioxide: Targeting Biofilms
There are methods available to assess the effectiveness of a disinfectant against a biofilm.
For wet biofilms, the MBEC assay (ASTM E2799) and the CDC model (ASTM E2871-22) can be used to assess disinfectant performance against biofilms of Staphylococcus aureus and Pseudomonas aeruginosa.
Tristel chlorine dioxide solutions have been assessed using these methods against biofilms grown over a 72-hour period. All chlorine dioxide solutions tested achieved ≥4 log reduction in the MBEC assay and ≥5 log reduction in the CDC model at their respective contact times, as short as 30 seconds. This rapid and robust activity is critical in real-world IPC workflows where time and efficacy must align.
For dry biofilms, chlorine dioxide’s performance was evaluated using the CDC model (ASTM E2871-22) modified using a 12-day dehydration/rehydration cycle. This approach is increasingly recognised for replicating real-world decontamination challenges posed by biofilms. All chlorine dioxide solutions tested achieved ≥5 log reductions at their respective contact times.
Implications for Infection Prevention
With chlorine dioxide proving effective against both surface contaminants and entrenched biofilms, its integration into routine IPC protocols could help tackle the challenge presented by biofilms and bridge the gap between compliance and real-world effectiveness.
Our high-level disinfectant solutions for medical devices:
Our sporicidal disinfection solutions for surfaces in healthcare:
Reference:
1 Ledwoch, K., Dancer, S.J., Otter, J.A., Kerr, K., Roposte, D., Rushton, L., Weiser, R., Mahenthiralingam, E., Muir, D.D. and Maillard, J.-Y. . (2018). Beware biofilm! Dry biofilms containing bacterial pathogens on multiple healthcare surfaces; a multi-centre study. Journal of Hospital Infection, 100(3), pp.e47–e56. doi:https://doi.org/10.1016/j.jhin.2018.06.028.
2 Maillard, J.-Y. and Centeleghe, I. (2023). How biofilm changes our understanding of cleaning and disinfection. Antimicrobial Resistance and Infection Control, [online] 12(1), p.95. doi:https://doi.org/10.1186/s13756-023-01290-4.
3 K Ledwoch, Vickery, K. and Maillard, J-Y. (2022). Dry surface biofilms: what you need to know. British journal of hospital medicine, 83(8), pp.1–3. doi:https://doi.org/10.12968/hmed.2022.0274.