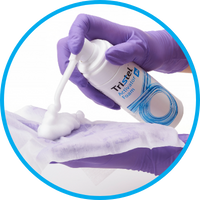
The complete device decontamination system
A comprehensive solution for the decontamination of semi-critical, non-lumened medical devices. It combines cleaning, high-level disinfection and rinsing with traceability.
Product Code: TSL030601
NHSSC: FAL14406

Applications
The Tristel Trio Wipes system is designed to decontaminate semi-critical, non-lumened medical devices such as:
Nasendoscopes
TOE/TEE Probes
Endocavity Probes
Laryngoscopes
Manometry Catheters
Ophthalmic Devices
Why Choose Tristel Trio Wipes System?
Tristel Trio Wipes streamlines decontamination processes into one workflow, which can be used at point of use and in a matter of minutes, helping to increase patient throughput.
- Effective
- Compatible
- Compliant
- Flexible
- Economical
-
The activated Tristel Sporicidal Wipe is a high-level disinfectant and is proven effective against a wide range of microorganisms in 30 seconds, including:
- Human papillomavirus (HPV)
- Coronaviruses
- Bacillus subtilis
- Clostridium sporogenes
- Mycobacterium tuberculosis
- Mycobacterium avium
- Aspergillus brasiliensis
- Candida albicans
- Adenovirus
- Staphylococcus aureus
- Herpes Simplex Virus type 1
For the full list of microorganisms Tristel Trio Wipes System is effective against, please see the Microbiological Efficacy Summary.
-
The system has been tested and proven to be compatible with the instruments of major manufacturers, including but not limited to,
- GE Healthcare
- Phillips
- Samsung Healthcare
- BK Medical
- Canon Medical Systems
- Siemens Healthineers
- Karl Storz
Check if your medical device is compatible with Tristel Trio Wipes System with our Compatibility Checker.
-
The Tristel Trio Wipes System has been independently tested and meets the requirements set out by European Norms including EN 14885. Sporicidal efficacy according to EN 17126:2018 is also demonstrated.
-
The Tristel Trio Wipes System is a mobile solution which can either be used in the consultation or decontamination room. Its flexibility eliminates the need for the instrument to be sent to central sterilisation units for reprocessing, saving valuable time.
-
The use of Tristel Trio Wipes System eliminates the investment in machines and maintenance contracts, allowing for significant cost savings. Tristel allows devices to be reprocessed quickly, lessening the need for purchasing many devices reducing capital expenditure.
Comprehensive Solution
By performing three wiping steps in sequence, the full decontamination procedure is completed.
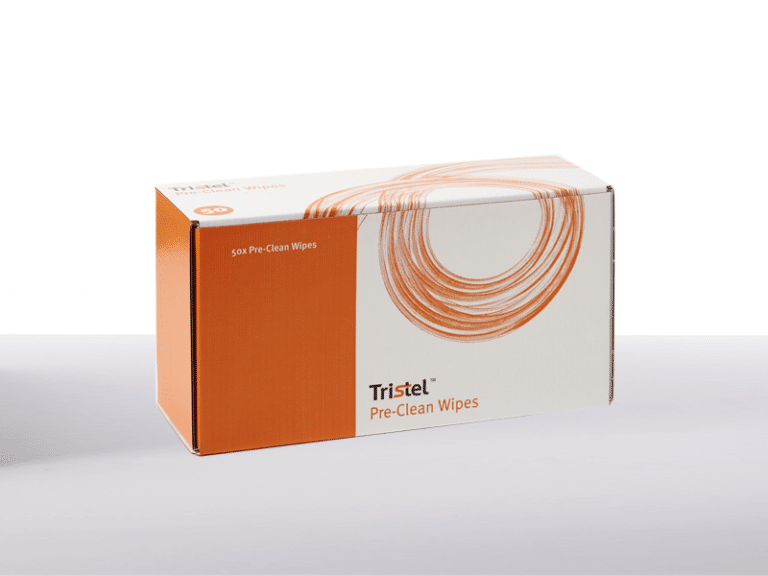
Cleaning
Tristel Pre-Clean Wipes
In order to ensure quick and effective cleaning, Tristel Pre-Clean Wipes are impregnated with a low-foaming surfactant and a triple-enzymatic detergent to remove organic matter.
Product Code: TSL030401
NHSSC: FAL510
High-Level Disinfection
Tristel Sporicidal Wipes
The Tristel Sporicidal Wipes combine with the Tristel Activator Foam to create our proprietary chlorine dioxide, a high-level disinfectant that eliminates microorganisms from the surface of a medical device in 30 seconds.
Product Code: TSL030201
NHSSC: FAL509


Rinsing
Tristel Rinse Wipes
The Tristel Rinse Wipes are impregnated with sterile water to remove any chemical residue following the high-level disinfection step.
Product Code: TSL030301
NHSSC: FAL581
Product Information
- Easy to Use
- Chemistry
- Resources
-
Easy to Use
Tristel Trio Wipes System consists of an Activator Foam and three different Wipes, when used in sequence, they perform the decontamination procedure.
Cleaning
The Tristel Pre-Clean Wipe is impregnated with a triple enzymatic detergent, to remove organic matter.High-level Disinfection
The Tristel Sporicidal Wipe combines with the Tristel Activator Foam to create chlorine dioxide, a high-level disinfectant that eliminates microorganisms from medical device surfaces in 30 seconds.Rinsing
The Tristel Rinse Wipe is impregnated with sterile water to remove any chemical residue from medical device surfaces after high-level disinfection.Traceability
Track your procedures using the Quality Audit Trail Record Book or use 3T, our digital traceability platform.
Refer to the User Guide for full instructions.Chemistry
The Tristel Sporicidal Wipe utilises our proprietary chlorine dioxide chemistry, a well-documented and highly effective biocide.
The Tristel Sporicidal Wipe is impregnated with Tristel Base Solution (citric acid) and the Tristel Activator Foam is a dilute solution of sodium chlorite. When mixed upon applying the Activator Foam onto the Wipe and scrunching together, our chlorine dioxide chemistry is generated.
Resources
-
Product Brochure
-
Microbiological Efficacy Summary
-
Device Compatibility
-
Wall Chart - Tristel Trio Wipes System with Cystoscope
-
Wall Chart - Tristel Trio Wipes System with TOE Probe
-
Wall Chart - Tristel Trio Wipes System with Skin Surface Probe
-
Wall Chart - Tristel Trio Wipes System with Manometry Catheter
-
Wall Chart - Tristel Trio Wipes System with Laryngoscopes
-
Wall Chart - Tristel Trio Wipes System with Flexible Nasendoscopes
-
Wall Chart - Tristel Trio Wipes System with Endocavity Ultrasound Probe - Cable, Plug, Holder
-
Wall Chart - Tristel Trio Wipes System with Endocavity Ultrasound Probe