Ultrasound Innovation: How Can We Keep Up?
Point of care ultrasound is bringing ultrasound to increasing numbers of hospital departments, practitioners and patients, with advancing technology making ultrasound more accessible than ever before, and an essential tool throughout the hospital. What is clear, is that ultrasound is changing, but can decontamination processes keep pace with these developments? Richard Fish discusses the challenges and solutions.
Point of care ultrasound (POCUS) is taking imaging to locations in hospitals where ultrasound would not have been found in years gone by. More than ever before, the modality is an essential diagnostic tool that can be found in medical imaging, obstetrics and gynaecology (OBGYN), cardiac surgery, the emergency department, vascular access, physiotherapy, ambulance services, the operating theatre, intensive care units (ICUs), and gastro-enterology; and this is by no means an exhaustive list. With POCUS, the ultrasound probe travels to the patient whenever and wherever care is needed. So, the traditional model of taking the patient to the ultrasound console is no longer the only patient pathway. Today, ultrasound can be delivered via an app and smart device technology: a mobile companion to the traditional console. Ultrasound is changing. But are Trusts’ decontamination processes able keep up?
 |
A key theme in ultrasound innovation is ‘static to mobile’, and disinfection of ultrasound equipment needs to stay on trend. Disinfection machines that are tied to a specific location, because they need a power supply or because they are only certified for use in that location by the staff that work there, are not going to keep pace with innovation in ultrasound.
Ultrasound is evolving rapidly. We’ve seen dramatic advances in the last decade and it’s an exciting and revolutionary field for practitioners. Rather than going to dedicated treatment rooms, what we are seeing is a greater reliance on the point of care approach. Ultrasound devices are becoming multi-use, wireless and mobile as new technology advances reach hospital front lines. Ultrasound is connecting with smart phones, being used in needle procedures, and providing manoeuvrable high-resolution imaging. The devices are travelling around hospitals, instead of diagnosis being anchored in one place. |
Making it mobile: can decontamination keep up?
POCUS may present a challenge to fixed location disinfection, but the widely accepted rules for the level of disinfection used for the ultrasound system should never be compromised and need not be. Throughout the United Kingdom, Europe, North America, and Australasia, in fact throughout most adequately resourced healthcare systems, it is recognised that a probe contacting mucosa, which includes intra-cavity probes used in vaginal and rectal examinations, require high-level disinfection. This is a term that is universally understood by infection prevention practitioners.
Fortunately, the medical device regulators in most countries set out very clear efficacy and safety criteria that a disinfectant must comply with to qualify as a high-level disinfectant. In Europe, the framework to support claims for microbiocidal activity is laid out in EN 14885. In Australia, the Therapeutic Goods Administration (TGA) governs the approval and ongoing regulation of disinfectants for intra-cavity probes. In the United States, the Food and Drug Administration (FDA) is the approval body.
So far, so clear – the disinfection standard for intra-cavity probes should not be compromised, and the good news is that there is choice for the ultrasound practitioner in where to turn to for their high-level disinfection ‘solution’.
There are disinfector machines using various biocidal technologies. A popular system uses hydrogen peroxide (H₂O₂) in the form of a mist; another uses ultra-violet light (UV-C); a number soak probes in liquids like peracetic acid (PAA). All these methods involve machinery which must incorporate a chamber that has an essential purpose: its walls must keep the disinfectant chemical or light rays from spilling out of the machine and into the room.
The dimension and shape of the chamber is designed to minimise the chemical needed to fill the chamber or the light rays needed to flood it, and these enclosures are only large enough to accommodate the probe. The cord and plug which connect the probe to the console will not fit into the machines and their chambers. The holder in which the probe is located on the console is also neglected. Published science confirms that the cord, plug and holder can all harbour pathogenic microorganisms that pose a risk to patient safety.
Ultrasound is in a constant state of evolution. Probe shapes and dimensions are changing. If the chambers of automated disinfectors cannot fit all of the probe in, then it can touch the side of the chamber. A consequence of this would be that the chemical mist or the light ray might not reach every surface of the probe. If an ultrasound manufacturer’s probe simply does not fit the chamber, an alternative high-level disinfection process must be purchased. In this instance, disinfector machines are not future proofed.
A final observation about machinery: it depends upon electricity to power it and requires continuous maintenance to function. It also requires capital investment to purchase.
An alternative to machinery is a wipe process that is performed by the practitioner and incorporates chlorine dioxide (ClO₂). This method has no space constraints so that the cord, and indeed the entire console including the probe holder, can be high-level disinfected. If there is to be no compromise, not only is high-level disinfection an absolute requirement for the infection prevention team, but so is the selection of a decontamination method that can address all surfaces of the ultrasound console.
There are many other wipe products available to hospitals, but they do not qualify as high-level disinfectants and are not approved as Class II Medical Devices like the ClO₂ system is. Low-level disinfectant wipes are not an acceptable option for intra-cavity ultrasound probes.
In considering the ultrasound decontamination options, the POCUS dimension is surely going to be increasingly important for the ultrasound practitioner and the infection prevention team. If the future direction of ultrasound innovation is toward mobile – not only in terms of ultrasound going to the patient, but also in terms of ultrasound technology moving to mobile devices and away from the fixed location console – the decontamination process needs to keep pace and be mobile too. In these scenarios, the high-level disinfection choice is limited to a mobile decontamination process. Only a manual process is mobile.
Following ‘the steps’
For the infection prevention profession, decontamination of medical devices such as ultrasound probes involves a sequence of steps; first, cleaning; second, disinfection; and in some cases, a third, rinsing. The conventional wisdom is that an instrument needs to be cleaned successfully if it is to be disinfected successfully. Only when both steps are carried out successfully can the required decontamination outcome – which is a high-level disinfected ultrasound probe, cord, and console surface – be assured. Cleaning is, therefore, an integral part of the decontamination process. So, what happens if the cleaning step is not successful?
The risk of an inadequate cleaning stage
Contamination on the surface of the probe – be it gel, soil of various types or even blood – after its use on the patient and handling by the ultrasound practitioner can compromise all the disinfection technologies referred to above. For the chemical disinfectants like H₂O₂, PAA and ClO₂, it is known that the active ingredients in the chemical formulation will react with the contaminants. What is left of the actives after they have chemically broken through the soil may render the disinfectant incapable of achieving the micro kill claims required for high-level disinfection. The successful removal of soil present on a medical device is the essential precursor for high-level disinfection by a chemical disinfectant.
For the UV-based high-level disinfection technology referred to above, soil on the instrument compromises successful disinfection in a different way. UV-C light rays, while proven very effective at destroying micro-organisms, can only be lethal if they reach the microbe. Soil becomes a shield – effectively an umbrella – protecting the virus, the bacterium, the spore from the UV-C light. While these machines can incorporate indicators and sensors that monitor the presence of UV-C light rays in the machine’s chamber, they cannot give any assurance that the rays have reached microbes on the ultrasound probe’s surface. The successful removal of soil present on a medical device is, therefore, an essential precursor for high-level disinfection by UV-C.
Cleaning products and high-level disinfection products that come from different stables cannot guarantee a successful decontamination outcome. It is beyond any reasonable expectation of infection prevention teams and ultrasound practitioners to judge that a combination of cleaning product and disinfectant can complement each other. Only scientific validation conducted in both laboratory and in use conditions can do this. If products come from different sources, the healthcare professional is forced to place their trust in multiple vendors’ product claims.
Surely, it would be best for the decontamination outcome if cleaning of the medical device is the responsibility of the provider of the disinfection step. Furthermore, each product used in the decontamination process must be approved by the manufacturer of the ultrasound equipment being decontaminated. Most of the decontamination options that are identified here do not provide a 360˚ solution for the infection prevention team and the ultrasound practitioner. Instead, they leave the healthcare team with the burden of piecing together a complete, compliant decontamination process, where all elements must be sanctioned by the ultrasound manufacturer. Industry could be doing more to help healthcare professionals do what they do best, and patients need most.
The need for a 360˚ ultrasound decontamination solution
We all recognise the importance of product training. The success of any decontamination process, whether it be machine-based or manual, is dependent upon the quality of the training provided. Machines have process steps; manual methods have process steps. Irrespective of the methodology, the steps need to be understood and adhered to by the user and the supplier has the responsibility to deliver the training required to ensure complete compliance.
However, if you throw multiple suppliers into the equation, the successful training outcome cannot be guaranteed. We also cannot ignore that decontamination products are medical devices – Class I for cleaning products and Class II for high-level disinfectants – and are governed in Europe by the Medical Device Directive, being succeeded by the Medical Device Regulation, and in the United Kingdom by the UKCA. The provider of a product or service that has multiple process steps will only fulfil the responsibilities placed upon it under its regulatory framework if it provides a 360˚ turn-key solution.
Furthermore, the dilemma is compounded as ultrasound goes mobile, via POCUS or technology. With POCUS, there can be no assurance that disinfection machines and scientifically validated, complementary cleaning products can arrive at the site of patient care on time and physically together for each, and every, ultrasound scan. If a mobile ultrasound product is being used, it surely needs a mobile decontamination product alongside it; mobile ultrasound devices are handheld, which suggests the decontamination product should be handheld also. The resounding takeaway is that, with the advances emerging in ultrasound, the accompanying high-level disinfectant product needs to be compact, lightweight, quick to set up and easy to use.
Rising to the challenge
Taking account of all the issues considered here, a manual decontamination process is the option best qualified to keep pace with ultrasound innovation. Two portable, ClO₂-based decontamination systems designed specifically for ultrasound are available in most countries.
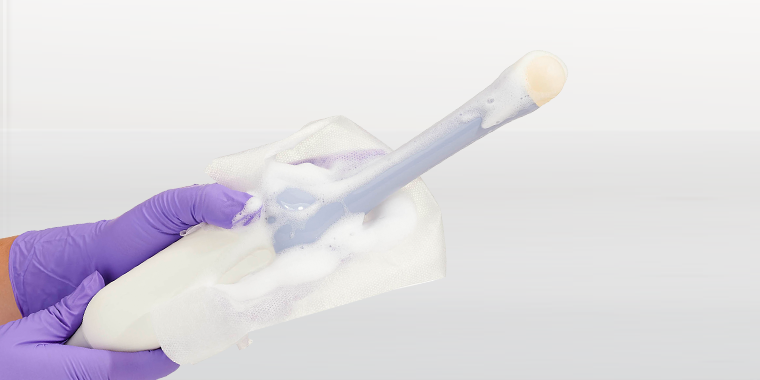
One of them incorporates cleaning, ClO₂ high-level disinfection, and rinsing in sequential steps that are each performed by a specialised wipe. Each wipe is applied by hand to the surface of the medical device and the entire process typically takes less than two minutes. The three wipes are contained in a compact lightweight box that can easily be carried by an individual from room to room and department to department. While rinsing is not necessary from the biocompatibility perspective, regulators often favour this third ‘belt and braces’ step which is why it is incorporated in the system.
The second system is a foam-based process that is more modular and can incorporate both cleaning and high-level disinfection steps. Interestingly, the ClO₂ foam meets all the efficacy challenges for high-level disinfection, even when soiling is present. This is thanks to the foam incorporating a surfactant to remove the soil, allowing the ClO₂ to perform its disinfection task unhindered. Both cleaning foam and the high-level disinfection foams are applied to the surface of the medical device by a dry wipe which is purposefully designed and validated to release the active ingredients onto the surface without loss of volume through absorption.
There is no getting away from wiping
 |
There are several high-level disinfection technologies and essentially two decontamination methods – machine or manual – for ultrasound equipment.
The infection prevention world recognises effective cleaning as the essential first step for high-level disinfection to be achieved. The vast majority of cleaning steps are performed with wipes. Consequently, the result is that there is no getting away from wiping in the decontamination process. Nonetheless, cleaning wipes all too often provide scant technical detail and little or no efficacy validation data. Frequently, they do not make clear (or users do not check) that they are appropriately classified for medical device use, and that they have been tested for compatibility by ultrasound manufacturers. Very few cleaning wipes have been performance tested in simulated use and real use conditions in combination with the disinfectant that follows them in the decontamination process. |
The selection of an optimum decontamination system for ultrasound really must start with the choice of cleaning wipe that is best supported by scientific data, regulatory compliance, and is accompanied by thoughtfully designed and continuous training. The best choice of system would be the one where the cleaning step and the high-level disinfection step come from the same supplier. This approach additionally enables the ultrasound practitioner and the infection prevention team to monitor and verify the process with a traceability and audit tool. The Tristel Trio and Duo systems supported by the 3T app compliance tool deliver all these features.
The Italian biologist Lazzaro Spallanzani first discovered ultrasound in 1794, and in 1942 Neurologist Karl Dussik was the first to use ultrasonic waves as a diagnostic tool. Since then, ultrasound technology has evolved through continuous innovation, and its application has expanded throughout the modern hospital. Decontamination technology must keep up with this innovation. As ultrasound increasingly goes to the point of use, providing significant benefits to hospitals and patients, the disinfection process must be able to travel with it.
About the author
 |
Richard Fish BEng Hons, a decontamination specialist, has worked in the medical device decontamination industry for over 18 years. Trained at Eastwood Park as a decontamination lead, and using his knowledge of national guidelines and regulations, he assists with audit preparation and hospital staff training, helping teams utilise the most effective ways to ensure complete device decontamination and infection control across departments and medical specialisms. Richard continuously develops his knowledge of the industry, with qualifications such as BSI ISO 13485 Medical Devices. He is an IPS Corporate Member and has a registered Medical industry accreditation (MIA) at Tier 3 and IntelliCentrics. Currently, Richard leads a team of ten decontamination specialists, ensuring they are also qualified to a high standard with Eastwood Park. |