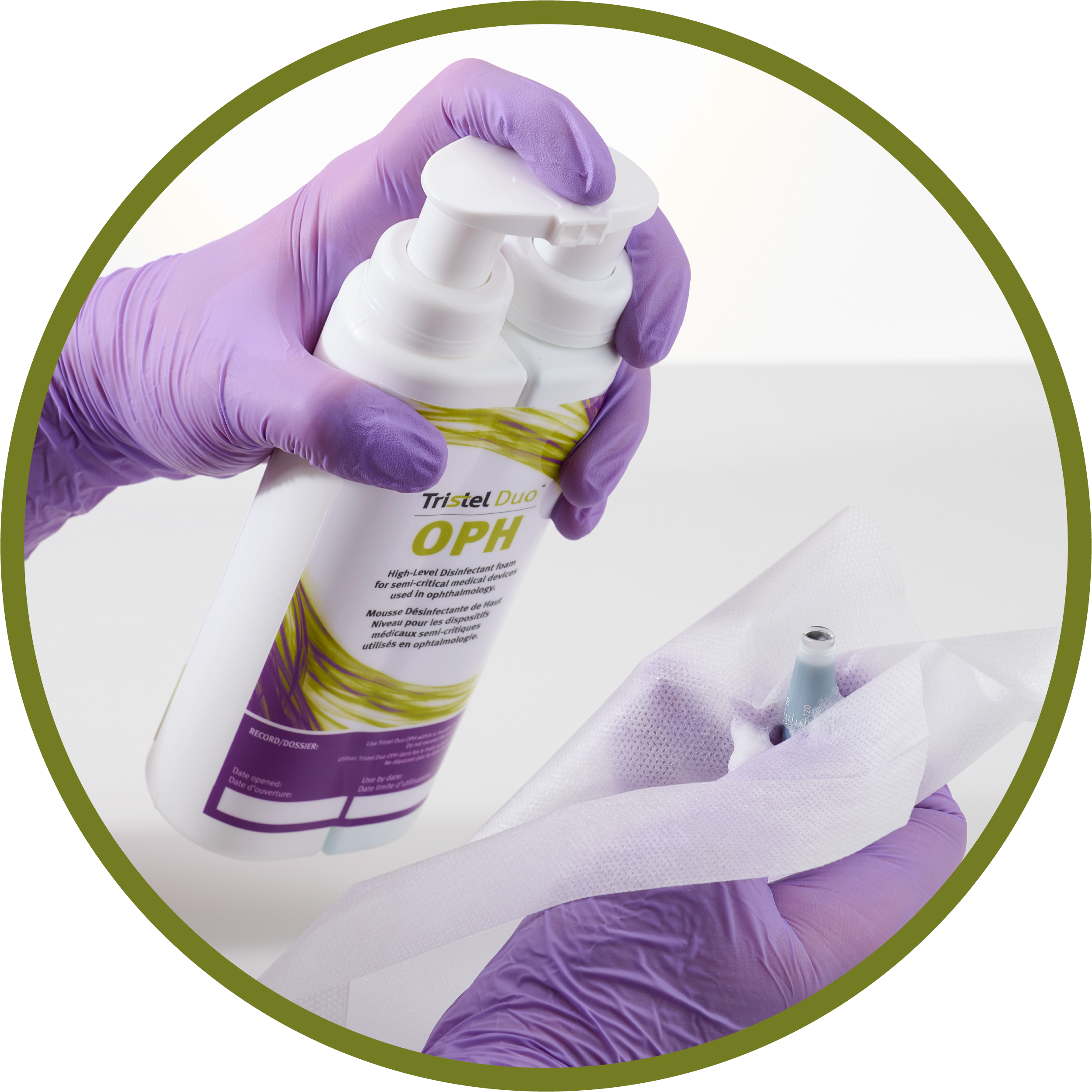
Effective, fast and compliant high-level disinfectant for ophthalmic devices
Achieve high levels of efficacy at a low concentration for ophthalmic and optometry medical devices.
Product Code: TSL023925

Applications
Tristel Duo OPH is used to high-level disinfect ophthalmic and optometry medical devices including:
Contact
Lenses
Tonometer Prisms
Ophthalmic A & B Scan Probe
Why Choose Tristel Duo OPH?
Within ophthalmology and optometry, clinicians are required to adhere to the highest level of infection prevention practices. Tristel Duo OPH offers effective, fast and compliant high-level disinfection solution for ophthalmic and optometry medical devices.
- Effective
- Compatible
- Mobile and Economical
- Compliant
-
Tristel Duo OPH is a high-level disinfectant and is proven effective against a wide range of microorganisms in 2 minutes, including:
- Acanthamoeba castellanii
- Adenovirus Type 5
- Aspergillus brasiliensis
- Fusarium solani
- Candida albicans
- Staphylococcus aureus
- Vancomycin-resistant Enterococci (VRE) Enterococcus faecium
- Carbapenem-resistant Enterobacteriaceae (CRE) Klebsiella pneumoniae
For the full list of microorganisms Tristel Duo OPH is effective against, please see the Microbiological Efficacy Summary.
-
Tristel Duo OPH has been tested and proven to be compatible with the instruments of major manufacturers, including:
- DGH Technologies
- Ellex
- Haag-Streit
- Keeler Accutome
- Natus Medical
- Nidek
- Quantel Medical
- Reichert Technologies
- Takagi
- Tomey
- Volk
Check if your medical device is compatible with Tristel Duo OPH with our Compatibility Checker.
-
The use of Tristel Duo OPH eliminates the need for single-use medical devices, allowing for significant cost savings. Tristel allows devices to be reprocessed quickly, lessening the need for purchasing many devices.
-
Tristel Duo OPH is authorized by Health Canada as a Class II medical device under the Canadian Medical Devices Regulations SOR/98-282.
Product information
- Description
- Easy to Use
- Chemistry
- Microbial Efficacy
- Traceability
- Resources
- Testimonials
-
Description
Tristel Duo OPH is a high-level disinfectant foam for use on cleaned, reusable, non-lumened, semi-critical devices used in ophthalmology.
The dual-compartment, ready-to-use bottle enables reprocessing ophthalmic instruments immediately and remotely, without the need for water or electricity.
Tristel Duo OPH has been tested in accordance with the latest and most stringent efficacy norms and is proven mycobactericidal, virucidal, fungicidal and bactericidal in 2-minute contact time.
The speed and practicality of Tristel Duo OPH allow for rapid instrument turnaround while reducing the need to purchase additional equipment, and contributes to a seamless, reliable and uninterrupted workflow.
Tristel Duo OPH carries a low toxicity rating and is not classified as hazardous according to CLP regulations, thus safeguarding the health of operators.
Tristel Duo OPH contains a non-ionic surfactant for added cleaning performance.
Each 250ml Tristel Duo OPH bottle provides 100 high-level disinfection events and is supplied in cases of two or six bottles. Each Tristel Duo OPH bottle has a shelf life of two years.
Tristel Duo OPH is authorized by Health Canada as a Class II medical device under the Canadian Medical Devices Regulations SOR/98-282.
-
Easy to Use
DispenseWipeWaitTraceRefer to the User Guide for full instructions.
-
Chemistry
Tristel Duo OPH utilizes our proprietary chlorine dioxide chemistry, a well-documented and highly effective biocide that destroys microorganisms by taking electrons, ensuring they cannot develop resistance to the chemistry.
It has two separate compartments that contain 125ml of Tristel Base Solution (citric acid) and 125ml of Tristel Activator Solution (sodium chlorite). When the pump is pressed, the two solutions mix and Tristel chlorine dioxide chemistry is generated.
Our chlorine dioxide does not form hazardous by-products or halogenated compounds.
-
Microbial Efficacy
Tristel Duo OPH is proven effective against bacteria, viruses, parasites, yeasts, fungi and mycobacteria in a contact time of 2 minutes.
For a complete list of micro efficacy data, refer to the Tristel Duo OPH Microbiological Efficacy Summary.
-
Traceability
Keep track of each disinfection procedure with Tristel 3T .
Tristel 3T is the free digital traceability and training system that makes tracking each disinfection procedure secure, fast, accurate and smart.
-
Resources
-
Testimonials
“We have been using Tristel Duo OPH for the disinfection of ocular lenses for 1 year. The product was proposed by the hygiene department of the University Hospital of Amiens. I like Tristel Duo OPH because it is practical, fast-acting, and easy to use in a foam format. The product is effective and provides high-level disinfection.”
- Amiens University Hospital, France – May 2021
“Staff find Tristel Duo OPH very easy to use. In our ophthalmics department the behaviours are very imbedded, and so we are happy with Tristel Duo OPH. There are no issues, and Tristel is fully accepted by the team.”
- Wirral University Teaching Hospital NHS Foundation Trust, UK – March 2022