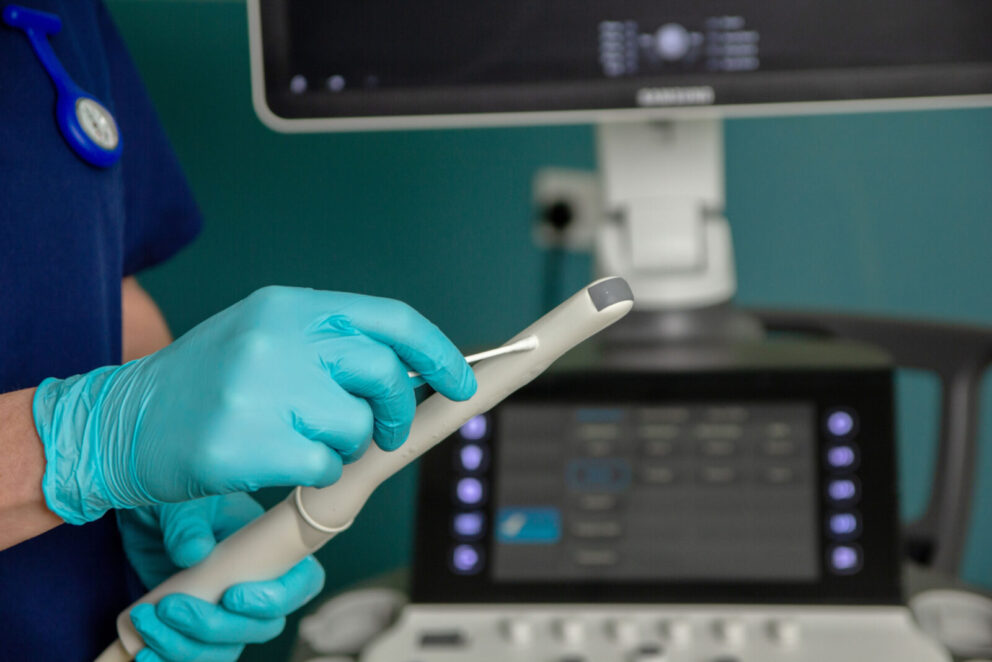
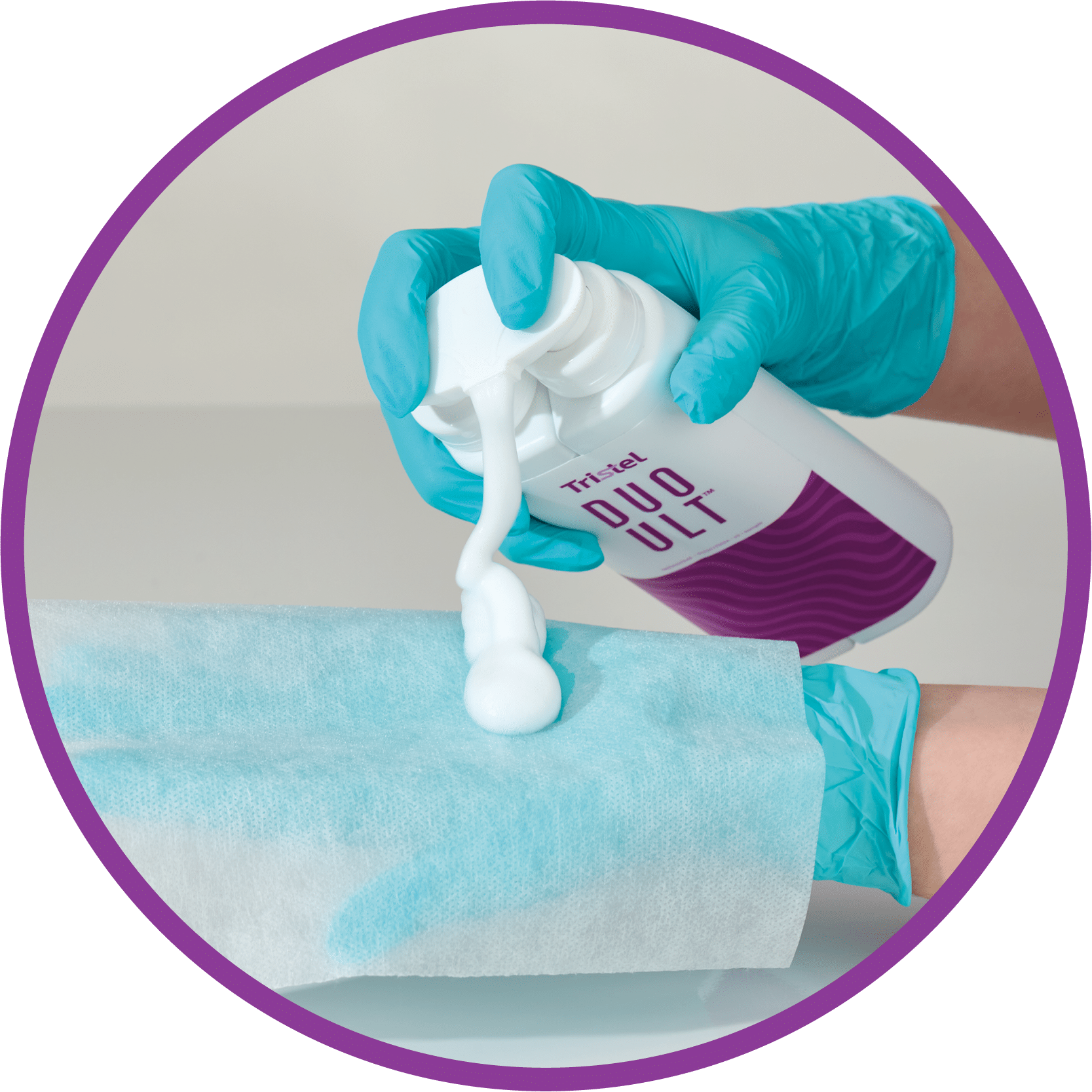
HPV effective high-level disinfectant for ultrasound
Offering high-level disinfection for all parts of an ultrasound transducer including the probe, holder, plug and cable.
Product Code: TSL022601
Available: Hong Kong, India, Malaysia, and Singapore

Same Product, New Look
Applications
High-level disinfection for all parts of an ultrasound transducer and station.
Endocavity
Probes
Skin Surface Transducers
Plug and
Cable
Ultrasound
System
Why Choose Tristel DUO ULT?
Tristel DUO ULT offers an effective, fast and compliant high-level disinfection solution for ultrasound devices.
- Effective
- Compatible
- Mobile and Economical
- Compliant
-
Proven effective against a wide range of microorganisms in 30 seconds, including:
- Clostridioides difficile
- Human Papillomavirus (HPV)
- Human Immunodeficiency Virus (HIV)
- Coronaviruses
- Herpes Simplex Virus (HSV)
- Candida albicans
- Gardnerella vaginalis
- Neisseria gonorrhoeae
- Staphylococcus aureus (MRSA)
- Streptococcus agalactiae
- Klebsiella pneumoniae (CRE & ESBL)
- Acinetobacter baumannii (MDRAB)
For the full list of microorganisms Tristel DUO ULT is effective against, please see the Microbiological Efficacy Summary.
-
Tested and proven to be compatible with the instruments of major manufacturers, including but not limited to:
- BD (Bard Access)
- Canon Medical Systems
- FUJIFILM Healthcare
- GE Healthcare
- Mindray
- Philips
- Samsung Healthcare
- Siemens Healthineers
- Sonoscape
- Verathon
-
Tristel DUO ULT can be used wherever required, without having to worry about the limitations that automated systems bring. Tristel DUO ULT is also suitable for use in remote locations where electricity and water are not readily available.
Tristel DUO ULT does not require investment in expensive equipment or service contracts. Two aliquots of foam and one Tristel DUO WIPE is all it takes to achieve disinfection to the highest level.
-
Independently tested to meet European Norm requirements within EN 14885 and the latest efficacy standards for the medical area.
It complies with guidelines for disinfection within ultrasound, such as BMUS, WFUMB and the Society for Maternal Fetal-Medicine.
It passes local regulatory requirements and is classified as Class IIb Medical Device.
Product Information
- Easy to Use
- Chemistry
- Resources
-
Easy to Use
DispenseOrder NowWipeWaitTraceRefer to the User Guide for full instructions.
Chemistry
Tristel DUO ULT utilises our proprietary chlorine dioxide chemistry, a well-documented and highly effective biocide that destroys microorganisms by taking electrons, ensuring they cannot develop resistance to the chemistry.
It has two separate compartments that contain 125ml of Tristel Base Solution (citric acid) and 125ml of Tristel Activator Solution (sodium chlorite). When the pump is pressed, the two solutions mix and Tristel chlorine dioxide chemistry is generated.
Our chlorine dioxide does not form hazardous by-products or halogenated compounds.
Resources