Désinfection révolutionnaire de haut niveau
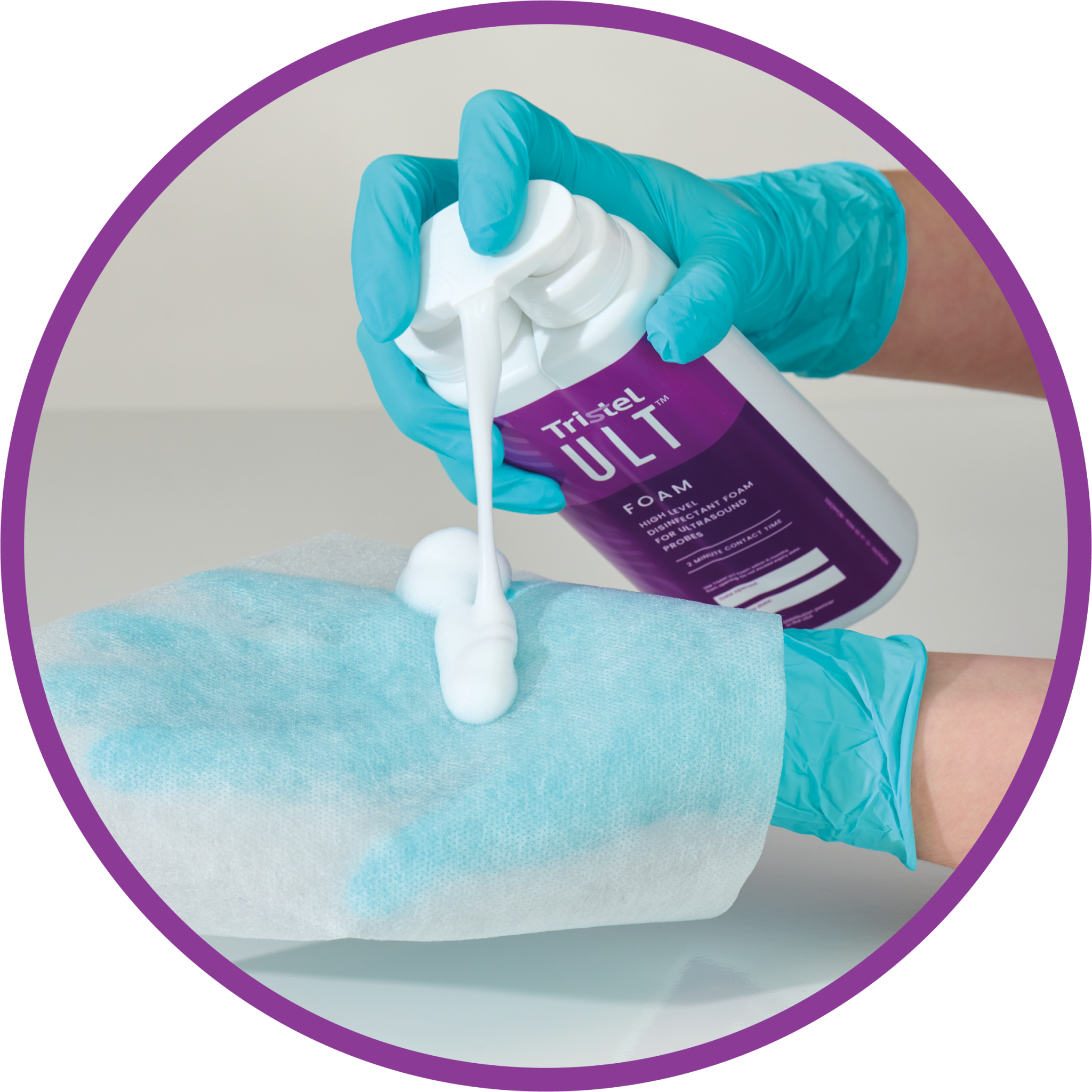
Obtenez une désinfection de haut niveau au point de service en seulement 2 minutes. Aucun équipement ni installation spécifique requis ; seulement quatre pressions de mousse CIO2 Tristel, approuvée par la FDA, sur une lingette sèche Tristel ULT.
Tristel ULT est compatible avec Tristel 3T, une plateforme numérique gratuite de traçabilité et de formation offrant un suivi fluide et une tranquillité d’esprit optimale.
Optimisez l’efficacité et le nombre de patients traités grâce à une solution économique et facile à utiliser. Compatible avec plus de 1 000 sondes d’échographie, Tristel ULT offre rapidité, efficacité et polyvalence.
Product Code: 46-25

Applications
Désinfection de haut niveau pour sondes à ultrasons.
Sondes
endocavitaires
Sondes de surface cutanée
Pourquoi choisir Tristel ULT?
Tristel ULT offre une solution de désinfection de haut niveau efficace, rapide et conforme pour les appareils à ultrasons.
- Efficace
- Compatible
- Mobile et économique
- Conforme
-
Efficacité prouvée en 2 minutes contre un large éventail de micro-organismes, y compris:
- Chlamydia trachomatis
- Neisseria gonorrhoeae
- Carbapenem resistant Klebsiella pneumoniae (CRKP)
- Extended-Spectrum beta-lactamase (ESBL) producing Escherichia coli
- Streptococcus agalactiae
- Candida albicans
- Human Immunodeficiency Virus (HIV) Type 1
- Hepatitis B Virus Surrogate Duck Hepatitis B Virus
Pour la liste complète des micro-organismes contre lesquels Tristel ULT est efficace, veuillez consulter le résumé d’efficacité microbiologique.
-
Testé et certifié compatible avec les dispositifs des principaux fabricants, y compris, mais sans s’y limiter:
-
- BD (Bard Access)
- Canon Medical Systems
- FUJIFILM Healthcare
- GE Healthcare
- Mindray
- Nipro
- Philips
- Samsung Healthcare
- Siemens Healthineers
- Sonoscape
- Sonosite
- Verathon
Vérifiez la compatibilité de votre dispositif médical avec Tristel Duo ULT grâce à notre vérificateur de compatibilité.
-
-
Tristel ULT offre une solution flexible, utilisable partout sans les contraintes des systèmes automatisés. Il ne nécessite ni électricité, ni eau.
Sans besoin d’investir dans des équipements onéreux ou des contrats de maintenance, une désinfection de haut niveau est assurée avec seulement quatre doses de mousse et deux lingette Tristel ULT.
-
Tristel ULT est classé comme dispositif médical de classe II en vertu du Règlement canadien sur les instruments médicaux DORS/98-282.
Informations sur le produit
- Description
- Comment utiliser
- Chimie
- Efficacité microbienne
- Traçabilité
- Ressources
- Où acheter
-
Description
Tristel ULT est une mousse désinfectante de haut niveau pour le retraitement des sondes d’échographie.
Tristel ULT peut être utilisé pour la désinfection de haut niveau des sondes endocavitaires transvaginales et transrectales, ainsi que des transducteurs cutanés susceptibles de toucher une peau non intacte pendant l’utilisation.
Tristel ULT est classé comme dispositif médical de classe II selon le Règlement canadien sur les instruments médicaux (DORS/98-282).
-
Comment utiliser
Distribuer
EssuyerTemps de contactTraçabilitéÉliminer les résidusConsultez le guide de l’utilisateur pour obtenir des instructions complètes.
-
Chimie
Tristel ULT utilise notre chimie exclusive au dioxyde de chlore (CLO2), organismes en prenant des électrons, garantissant qu’ils ne peuvent pas développer de résistance à la chimie.
Il dispose de deux compartiments séparés contenant 125 ml de solution de base Tristel (acide citrique) et 125 ml de solution d’activation Tristel (chlorite de sodium). En appuyant sur la pompe, les deux solutions se mélangent et le dioxyde de chlore Tristel est généré.
Notre dioxyde de chlore ne produit pas de sous-produits dangereux ni de composés halogénés.
-
Efficacité microbienne
Tristel ULT est un désinfectant de haut niveau avec un temps de contact de 2 minutes. Son efficacité contre les mycobactéries, les virus, les champignons et les bactéries a été testée. Tristel ULT a réussi le test d’activité sporicide de l’AOAC.
Des tests spécifiques ont été effectués sur les microbes cliniquement pertinents.
L’efficacité de Tristel ULT a été testée contre les papillomavirus humains de type 16 (HPV16) et de type 18 (HPV18) dans le cadre d’une étude menée par le Dr Craig Meyers au Pennsylvania State College of Medicine, aux États-Unis. L’étude conclut que Tristel ULT inactive efficacement les VPH16 et 181.
Études de dispositifs simulés avec Mycobacterium terrae efficacité validée sur les sondes à ultrasons. Des études cliniques en cours d’utilisation dans des hôpitaux américains ont confirmé l’efficacité du Tristel ULT en milieu clinique, aucun organisme n’ayant été retrouvé à partir des sondes à ultrasons contaminées par les patients et désinfectées à haut niveau avec du Tristel ULT.
1Meyers C, Milici J, Robison R. The ability of two chlorine dioxide chemistries to inactivate human papillomavirus-contaminated endocavitary ultrasound probes and nasendoscopes. J Med Virol. 2020 Aug;92(8):1298-1302. doi: 10.1002/jmv.25666. Epub 2020 Feb 4. PMID: 31919857; PMCID: PMC7497195.
-
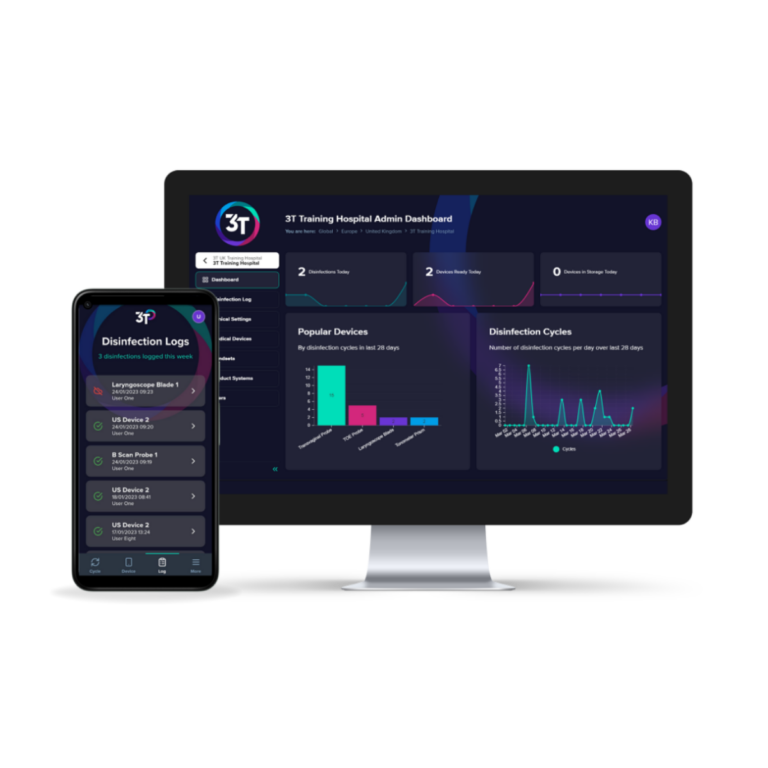
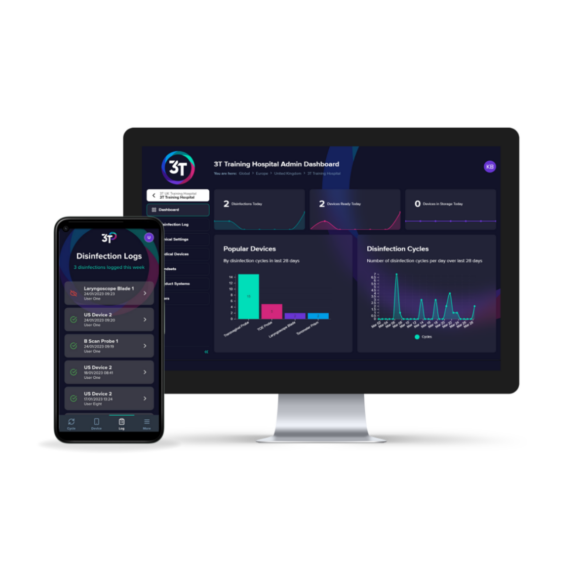
Traçabilité
3T est une plateforme de conformité numérique facile à utiliser qui vous permet d’enregistrer les cycles de désinfection de haut niveau et de suivre la formation du personnel au même endroit.
3T combine une application mobile intuitive, utilisable au point de service, avec un portail Web interactif. Elle offre aux utilisateurs un outil précieux pour l’enregistrement et la traçabilité du retraitement des dispositifs.
3T guide les utilisateurs tout au long des étapes de tenue des dossiers des appareils, en suivant chaque événement et en rendant le dossier consultable en temps réel.
Les données agrégées des événements de retraitement des appareils sont stockées de manière sécurisée et sont accessibles via des tableaux de bord interactifs.Pour la traçabilité manuelle, une feuille de journal est téléchargeable.
-
Ressources
-
Où acheter
CONTACTER LES LABORATOIRES PARKER
Email: customerservice@parkerlabs.com – Appel: 973.276.9500CONTACTER INNOVA MEDICAL OPHTHALMICS
Tristel ULT est également disponible auprès de votre fournisseur de confiance de Tristel OPH, INNOVA Medical Ophthalmics. Pour toute demande de renseignements, veuillez communiquer: contact@innovamed.comTRISTEL ULT
TRANSPORT
Tristel ULT n’est pas dangereux pour le transport et peut être transporté par voie aérienne, routière et maritime sans restrictions.