Why cleaning your ultrasound device before high-level disinfection is so important.
Cleaning is the first stage in any decontamination process. Before disinfecting an ultrasound device, it is vital to include a cleaning stage to remove soiling or visible dirt.[1] This soiling is formed of organic and inorganic materials, and is either visible to the naked eye or by peeking through a microscope. In real terms, soiling could contain ultrasound gel, bodily fluids, or even blood. A wipe impregnated with an enzymatic detergent is typically used for ultrasound device cleaning.
Working from the least amount of soiling to the most, all parts of the device should be wiped clean, ensuring to include the plug, cable and probe holder. Paying particular attention to device indentations; use a wipe or cotton swab to cover any hard-to-reach places where soiling could accumulate.
The action of manually applying chemistry with a wipe can aid the overall efficacy of the process. It manually controls where the chemistry goes and gives multiple opportunities to remove soiling. As a result, practitioners can ensure all contamination can be eliminated and prevent the build-up of hardened soiling on the device over time.
What if the cleaning stage is imperfect?
An imperfect cleaning stage can leave residual soiling on your device. Here, soiling may act as a protective layer for harmful microorganisms.
In this instance, the layer of soiling inhibits the disinfectant. It prevents complete disinfectant coverage on your device and limits the effectiveness of your high-level disinfection (HLD) stage. The result of this imperfect cleaning then creates a greater risk of contamination for yourself and your patients.
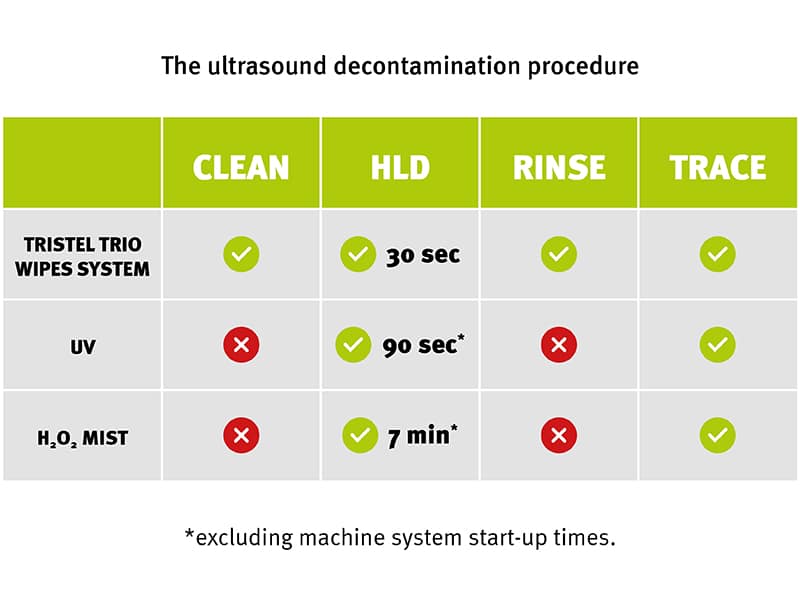
Residual soiling becomes particularly problematic when using automated methods such as UV and H₂O₂-based systems. Unlike a wiping method, high-level disinfecting in these automated systems involves no mechanical action at all, which can make an imperfect cleaning stage even more difficult to overcome.
As automated systems do not incorporate a cleaning step, practitioners must search for their own solutions that could prove to be incompatible. Moreover, as automated systems do not treat the entire device, the plug, cable and probe holder remain untouched and potentially contaminated. [2] The result is that the ultrasound device can be hiding pathogens of concern ready for the next patient.
The cleaning checklist for your ultrasound device:
 Ultrasound device compatibility:
Ultrasound device compatibility:
It is important that the products used on your ultrasound device are compatible. The wrong cleaning products can be overly corrosive on your device. For this reason, incompatibility can lead to micro-cracks and breakages in the device, capable of harbouring harmful microorganisms. When treating your semi-critical medical devices, checking your cleaning product is in date, and ensuring it is categorised as a Class I Medical Device (CE marked) are two ways to ensure your ultrasound device cleaning product is suitable to perform the task at hand.
 Preventing biofilm formation:
Preventing biofilm formation:
Be sure to complete the cleaning stage immediately after probe use. Gels and organic matter can quickly harden and form a film on your ultrasound device, making it harder to clean. Completing the cleaning stage quickly and thoroughly can help prevent contaminants from sticking to your ultrasound device.
 A sheath alone is not enough to prevent infection:
A sheath alone is not enough to prevent infection:
Published science shows that despite offering protection, sheaths are no substitute for a complete decontamination procedure. [3] [4] [5] To limit cases of cross-contamination, it is vital to use a system that incorporates all the decontamination stages: cleaning, high-level disinfection, and rinsing.
 Track and trace:
Track and trace:
Using a manual or digitalised track and trace system for each stage of your decontamination procedure covers your process by documenting the specific device and products used. So, for audits, investigations, and general peace of mind, your steps are made easy to check.
Tristel’s decontamination solutions for ultrasound
When it comes to ultrasound decontamination routines, having a system that incorporates a cleaning stage is essential to achieving effective high-level disinfection. No matter the method of decontamination, it is clear that wiping works for every single one. What’s more: all manufacturers are 100% reliant upon wiping.
Tristel provides fast, portable, and reliable decontamination solutions designed specifically for ultrasound.
Both the Tristel Trio Wipes System and the Tristel Duo ULT System go several steps further than other systems, delivering complete decontamination for the entire ultrasound device in a matter of minutes. Ticking all the boxes when it comes to efficacy, portability, compatibility, and traceability, the Tristel Trio Wipes System and Duo ULT System are designed specifically to keep pace with the expanding use of ultrasound, while always complying with official decontamination guidelines.
To find out more about perfecting your ultrasound decontamination procedures, as well as discovering the best solution for your requirements, contact our team of experts today.
[1] Centres for Disease Control and Prevention (CDC), Cleaning, Guideline for Disinfection and Sterilisation in Healthcare Facilities (2008)
[2] Health Technical Memorandum 01-06: Decontamination of flexible endoscopes. Part C: Operational management (england.nhs.uk)
[3]Rooks VJ, Yancey MK, Elg SA, Brueske L. Comparison of probe sheaths for endovaginal sonography. Obstet Gynecol. 1996;87(1):27-29.
[4] Basseal JM, Campbell Westerway S, Hyett JA, 2020, Analysis of the integrity of ultrasound probe covers used for transvaginal examinations – PubMed (nih.gov)
[5] Hignett M, Claman P. High rates of perforation are found in endovaginal ultrasound probe covers before and after oocyte retrieval for in vitro fertilization-embryo transfer. J Assist Reprod Genet. 1995;12(9):606-609.